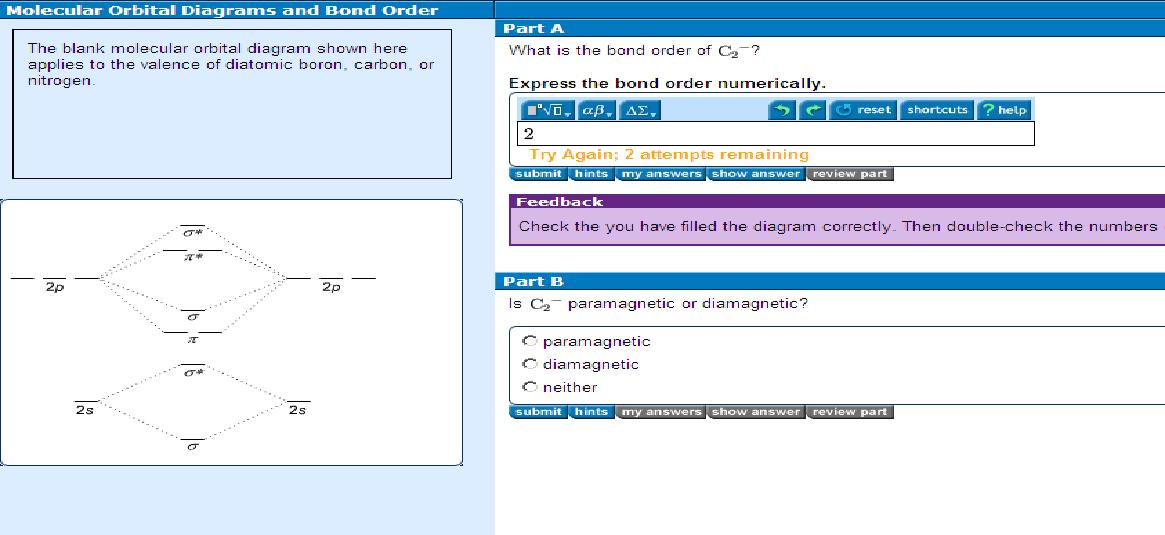
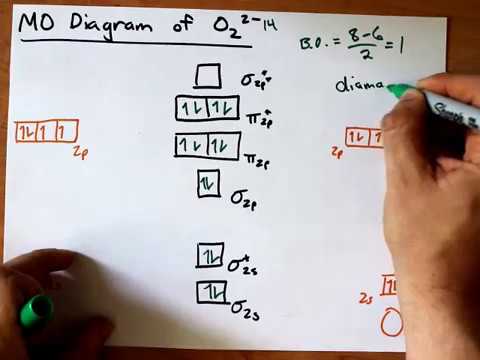
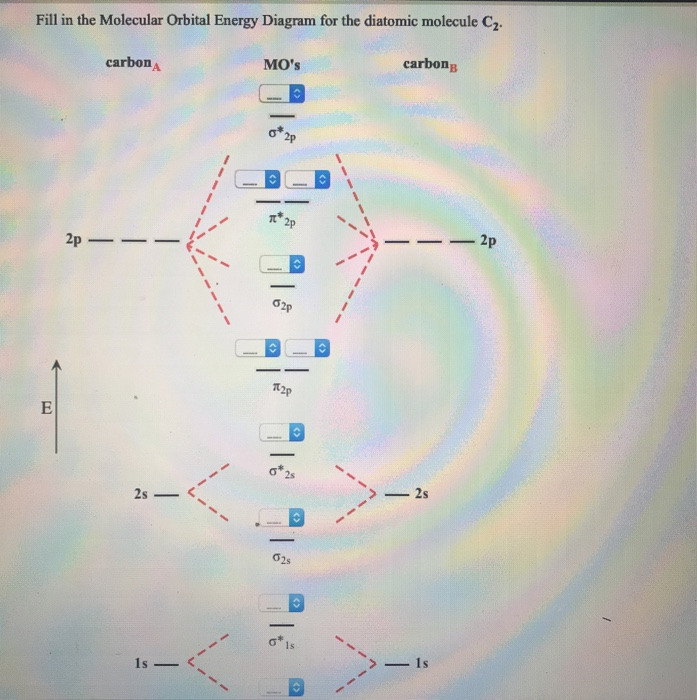
37 molecular orbital diagram for c2 2-
Use MO diagram to place C2-,C2 and C2+ in order of decreasing bond energy? Use MO diagram to place C 2- ,C 2 and C 2+ in order of decreasing bond energy? Q. Using the molecular orbital model, write electronic configurations for the following diatomic species and calculate the bond orders. a. Molecular Orbital C2- Diagram, Bond Order, Magnetism. Postby Brooke Tobias 1B » Fri Nov 27, 2015 7:31 pm. A video explaining the molecular orbital diagram and notation of C2- along with the bond order and magnetism. You do not have the required permissions to view the files attached to this post. Top.
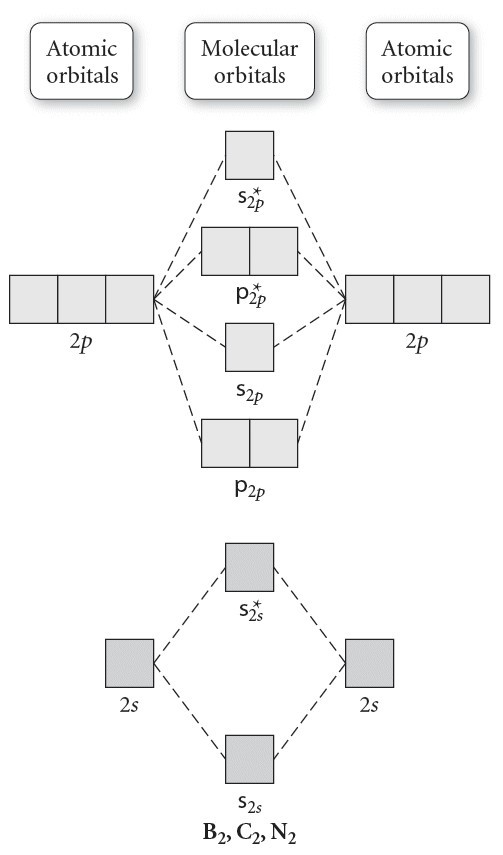
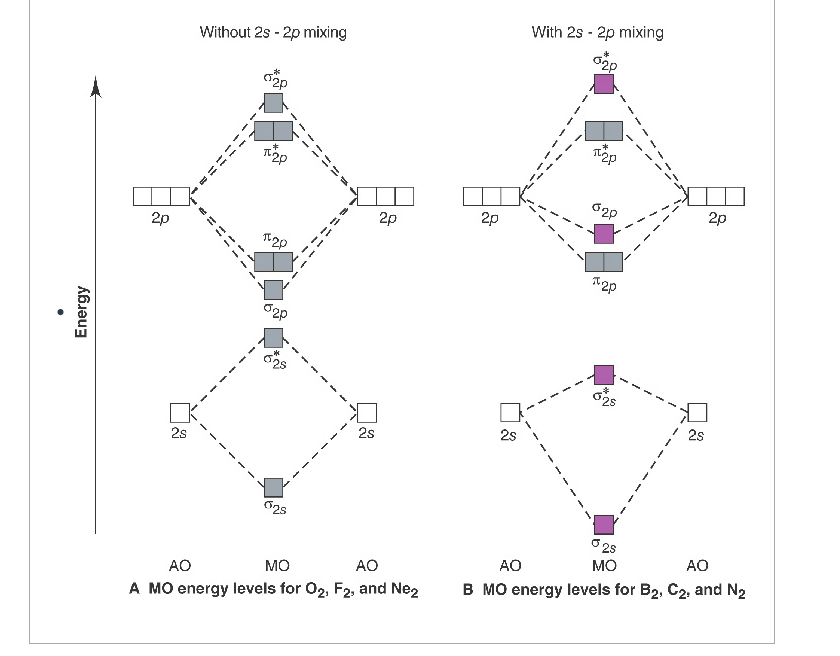
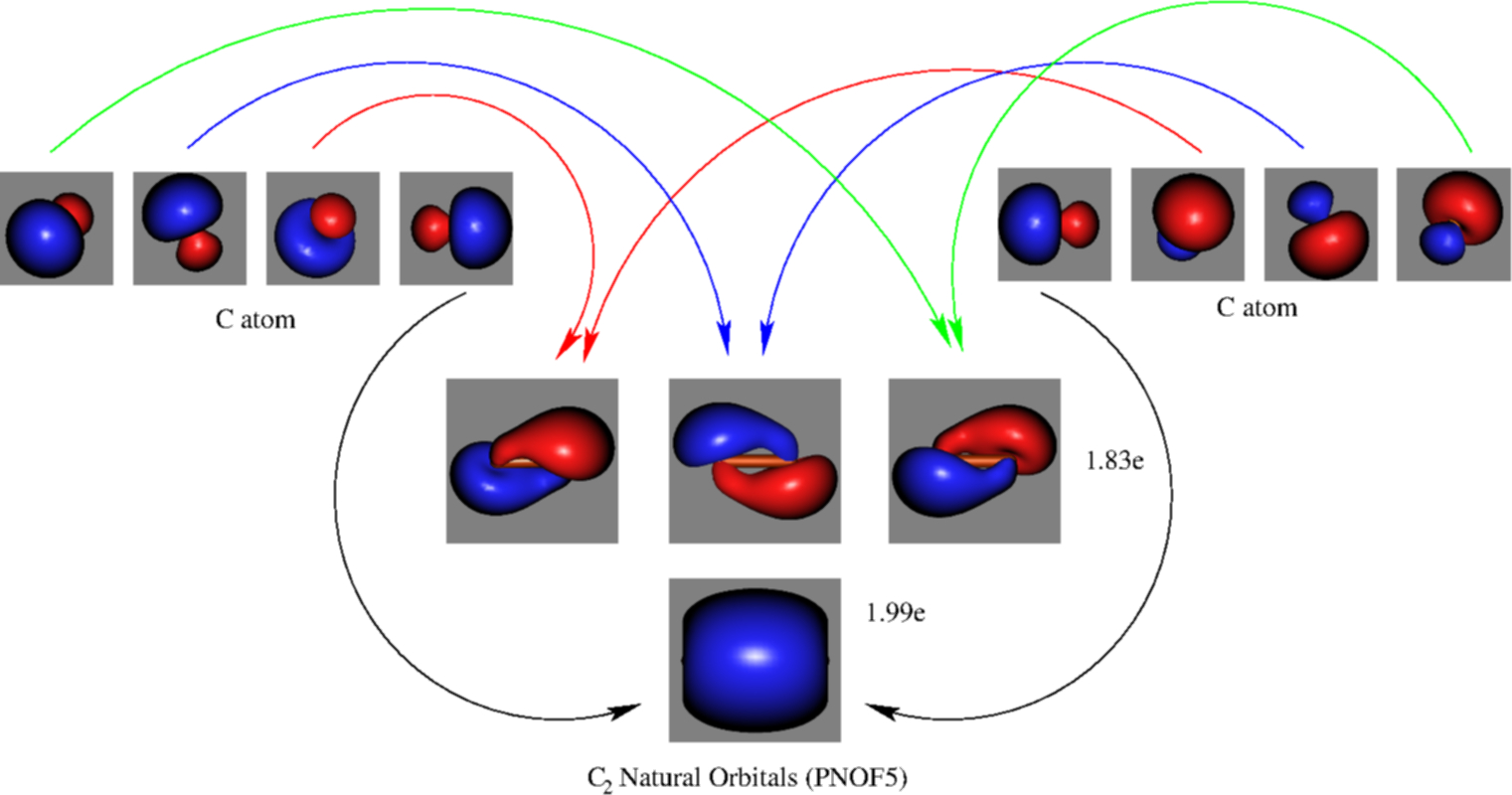
Molecular orbital diagram for carbon dimer c2. For the ion c22. Give the molecular orbital configuration for the valence electrons in cec22. A draw the molecular orbital diagram. N 2 has a bond order of 3 and is diamagnetic. Bonding order is 2 and it is diamagnetic. Interact and form molecular orbitals. B calculate the bond order.
Molecular orbital diagram for c2 2-
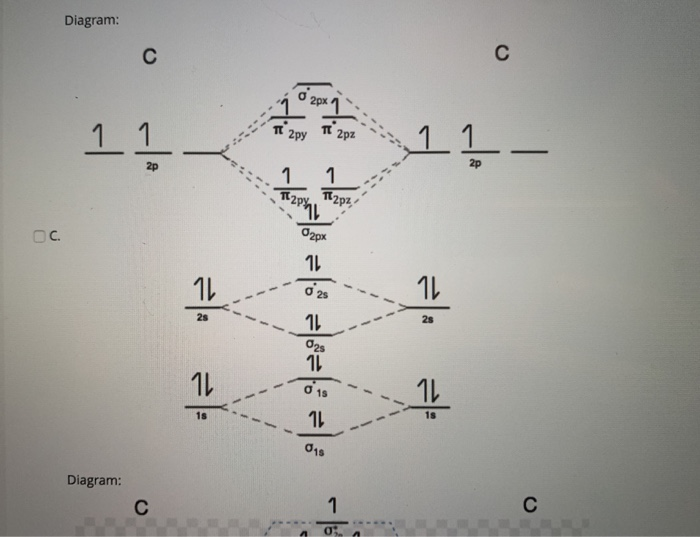
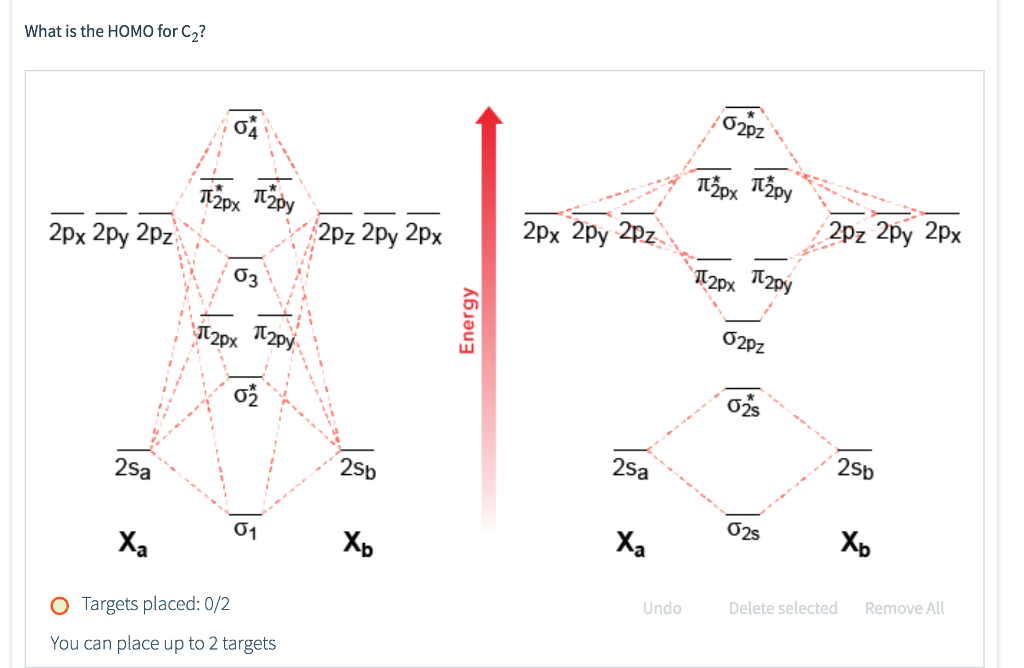
5. Draw Molecular Orbital Diagrams for C2, C2 and C2. a. Determine the bond order for each molecule b. Determine whether each of the molecules is paramagnetic ...1 answer · 0 votes: 646 -12 es IS IS LS IS 一4 Bond.ng-anhtond.ra elachroro Dia mame 12-1)ニ1 Peoamagnefr Anamngnetて The molecular diagram you would use is the Z<8 one because carbon's atomic number is 6, however when filling in the valence electrons you would use 10 because it is two carbons (C2) (4+4) with a negative 2 charge so (+2 valence electrons). The bond order of this would be calculated using the middle pi and sigma bonds. Molecular orbital diagram for n2 o2 c2 f2 also h2o. Electronic configuration of c2 molecule is σ 1s2 σ1s2 σ2s2 σ2pz 2 2px 1 2py orbitals what is the origin of differences between mo schemes o₂ and n₂ chemistry stack exchange c22 lewis structure how to draw the for c2 2 youtube figure orbital correlation diagram for homonuclear diatomic ...
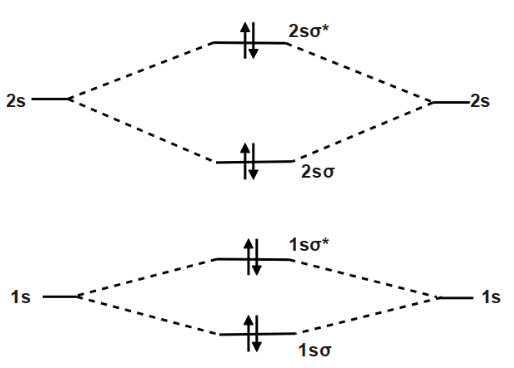
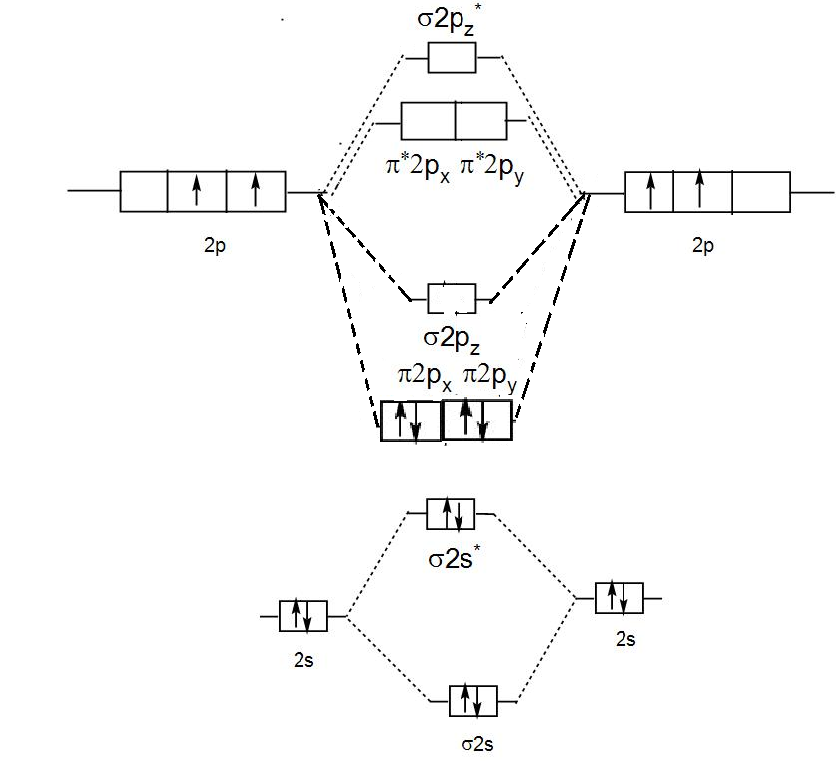
Molecular orbital diagram for c2 2-. Therefore determination of the bond order should explain the differences in the bond length. Solution. Molecular orbital energy level diagram for C2. 2–. : ...24 pages 13 Jun 2021 — Answer. Use molecular orbital theory to describe the bonding in the following. For each one, find the bond order and decide whether it is ...4 answers · Top answer: So here we're looking at the molecular orbital theory to describe bonding. So the first example, ... The lowest energy unoccupied molecular orbital is 2pσ, so that is where the extra electron will be added. The electron configuration of the neutral C2 molecule is -- I'll use the notation given to you in the diagram. C2:(1sσ)2(1s* σ)2(2sσ)2(2s* σ)2(2pπ)4. The electron configuration of the C− 2 ion will be. 6:46For the ion C22+: a) Draw the molecular orbital diagram. b) Calculate the bond order. c) Would this ion ...5 Apr 2013 · Uploaded by Professor Heath's Chemistry Channel
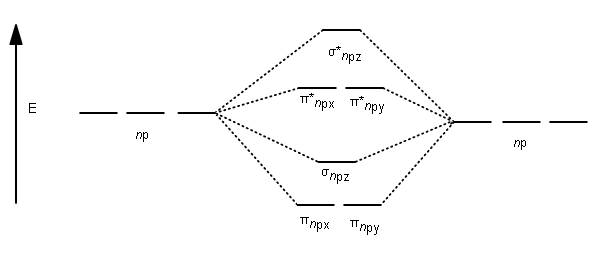
When two carbons atoms bond, the pi(2p) bonding molecular orbitals are lower in energy than the sigma(2p) bonding orbitals.C2(2-) has a bond order of 3, so i... 10.8K answers. 116M people helped. Bond order = 1/2 (number of electrons in bonding orbitals - number of electrons in antibonding orbitals) Therefore, Bond order of C2+ = 1/2 (5 - 2) = 3/2 = 1.5. Bond order of C2- = 1/2 (7 - 2) = 5/2 = 2.5. Bond order of C2 = 1/2 (6 - 2) = 2. Highest bond order means highest bond energy and shortest bond length ... This video discusses how to draw the molecular orbital (MO) diagram for the C2(2-) molecule. The bond order of C2(2-) is also calculated and the meaning of t... The Molecular Orbital Diagram For C2^2-Question: The Molecular Orbital Diagram For C2^2-This problem has been solved! See the answer. the molecular orbital diagram for C2^2-Expert Answer 100% (3 ratings) Previous question Next question ...
Molecular orbital diagram for n2 o2 c2 f2 also h2o. Electronic configuration of c2 molecule is σ 1s2 σ1s2 σ2s2 σ2pz 2 2px 1 2py orbitals what is the origin of differences between mo schemes o₂ and n₂ chemistry stack exchange c22 lewis structure how to draw the for c2 2 youtube figure orbital correlation diagram for homonuclear diatomic ... The molecular diagram you would use is the Z<8 one because carbon's atomic number is 6, however when filling in the valence electrons you would use 10 because it is two carbons (C2) (4+4) with a negative 2 charge so (+2 valence electrons). The bond order of this would be calculated using the middle pi and sigma bonds. 5. Draw Molecular Orbital Diagrams for C2, C2 and C2. a. Determine the bond order for each molecule b. Determine whether each of the molecules is paramagnetic ...1 answer · 0 votes: 646 -12 es IS IS LS IS 一4 Bond.ng-anhtond.ra elachroro Dia mame 12-1)ニ1 Peoamagnefr Anamngnetて

Assuming The Mo Diagram Above Would Apply To Both Species Predict How The Bond Order Will Change Upon Adding An Electron To Cn To Form Cn The Bond Order Will Increase Decrease
Solved 5 Draw Complete Molecular Orbital Diagrams To Compare The Bonding In C2 F2 And Cf A What Is The Bond Order Of Each B Which Of The Thre Course Hero
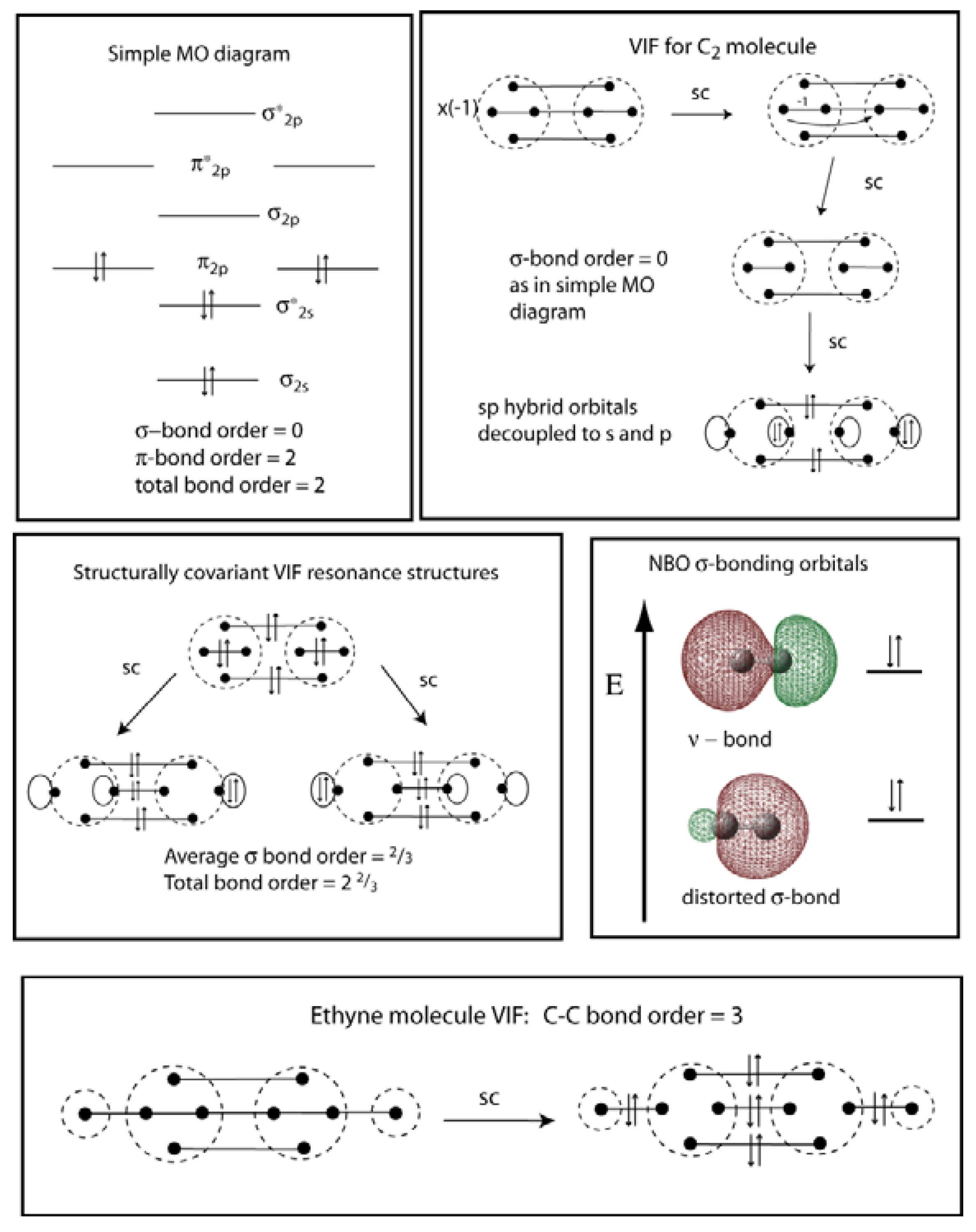
Symmetry Free Full Text Chemical Reasoning Based On An Invariance Property Bond And Lone Pair Pictures In Quantum Structural Formulas Html


















0 Response to "37 molecular orbital diagram for c2 2-"
Post a Comment