37 orbital diagram for scandium
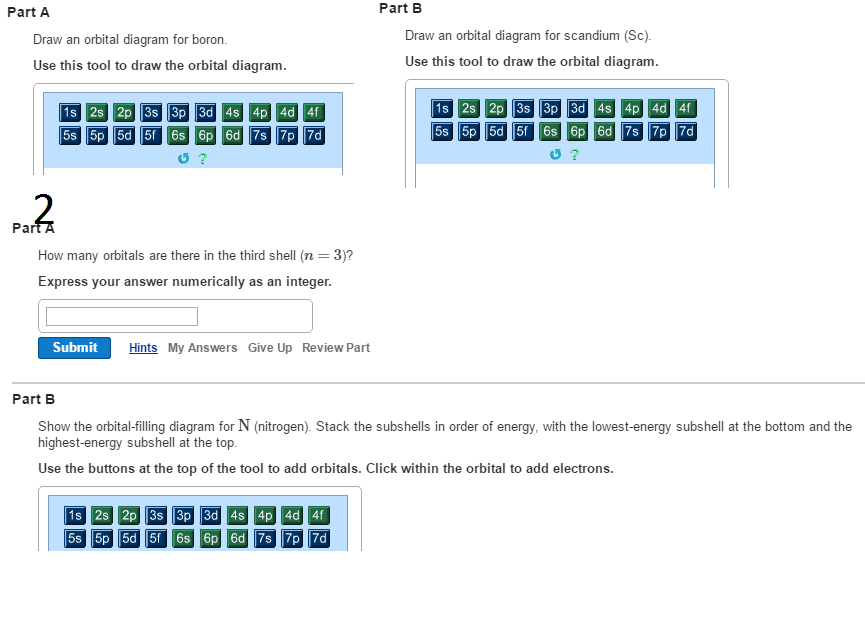
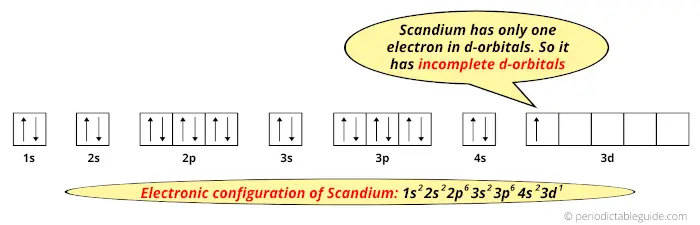
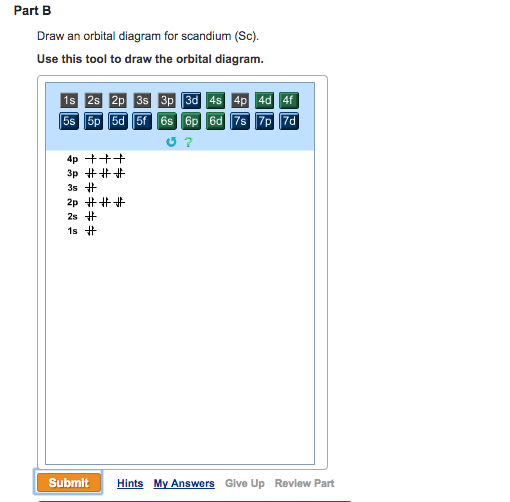
Draw an orbital diagram for scandium (Sc). Use this tool to draw the orbital diagram. How many orbitals are there in the third shell (n = 3)? Express your answer numerically as an integer. Show the orbital-filling diagram for N (nitrogen). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest ... Answer (1 of 10): Scandium has an atomic no. of 21. Therefore electronic configuration of scandium(Sc) is: 1s2 2s2 2p6 3s2 3p6 3d1 4s2. Here, two electrons are in 4s orbital instead of being in 3d orbital because 4s orbital is having lesser energy than 3d orbital and 4s orbital also gives full...
Orbital Diagram For Arsenic. Written By Pelvic Diagram Wednesday, February 24, 2021. Edit. Orbital Diagram For Arsenic. Draw an orbital diagram for scandium ScDraw an orbital diagram for scandium (Sc. A: The most stable conformation of cyclohexane is the chair. Electron Configuration for Arsenic (33 electrons); Quantum ...

Orbital diagram for scandium
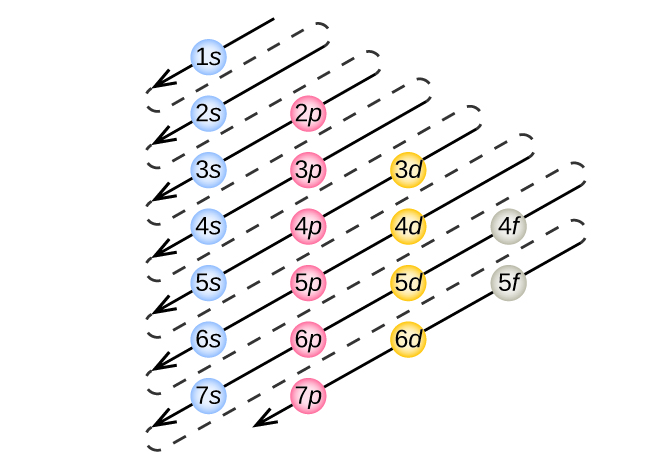
So for scandium the 1st and 2nd electron must be in 1s orbital, the 3rd and 4th in the 2s, the 5th through 10th in the 2p orbitals, etc. You are watching: Enter the orbital diagram for the ion au+. This is a memory device to remember the order of orbitals for the first two quantum numbers. Follow the arrow starting in the upper right, when the ... Complete an orbital diagram for scandium sc. Label all bonds in bf3. Label all bonds in bf3. Label each bond in the molecules as polar or nonpolar and give the shape of each molecule and describe whether each molecule and tell whether each is soluble or insoluble in water. The three sp2 hybrid orbitals have a trigonal planar arrangement to ... The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = +1 2 m s = + 1 2 ).
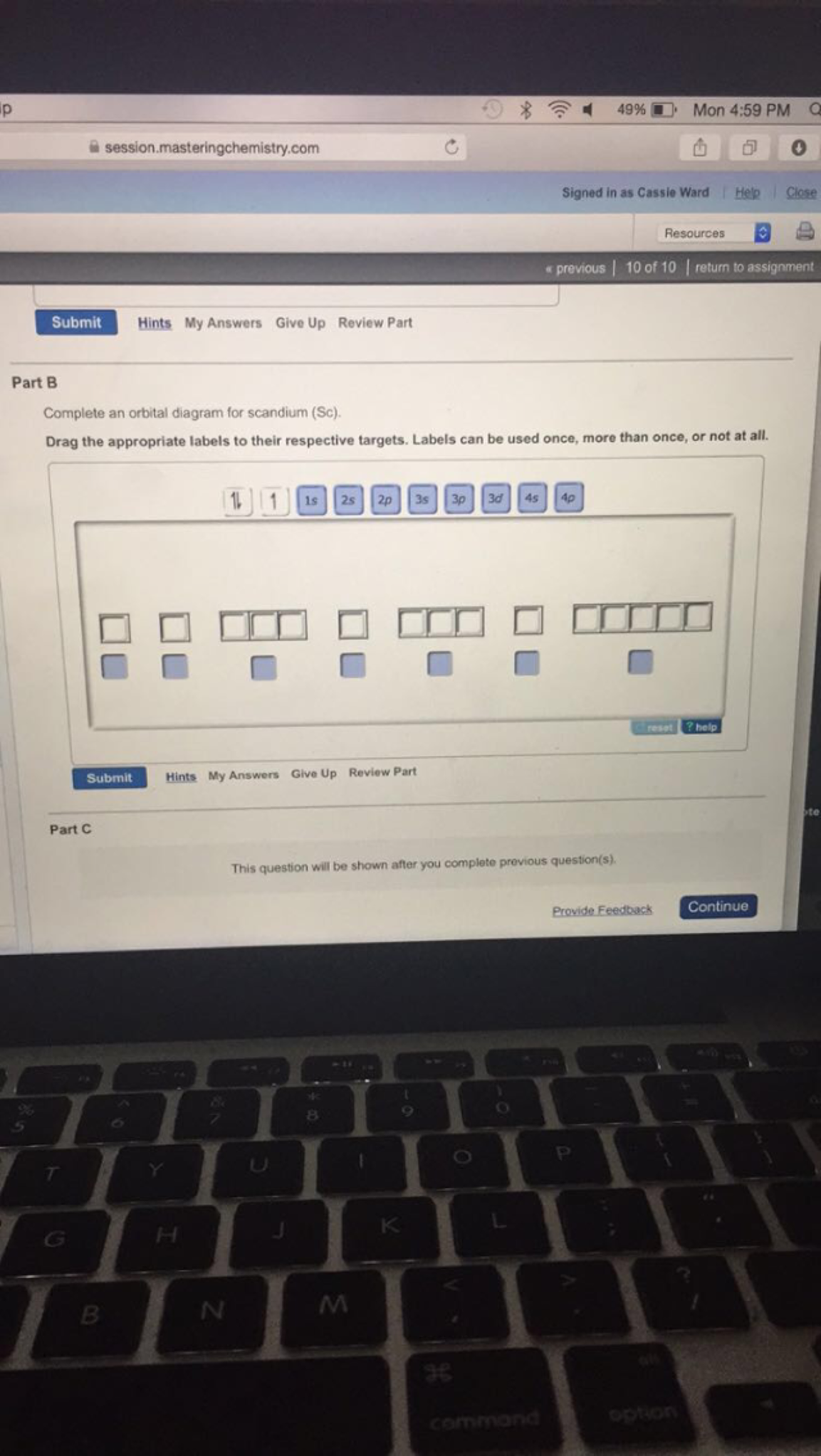
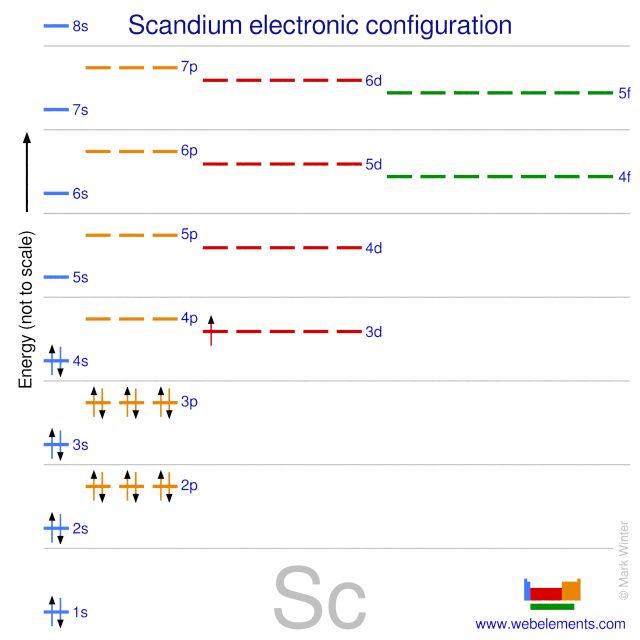
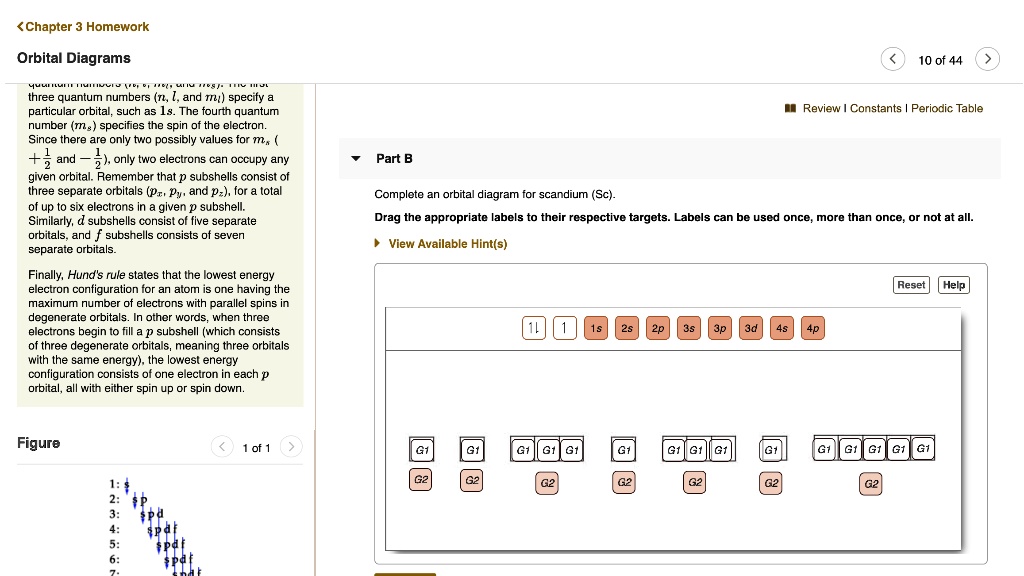
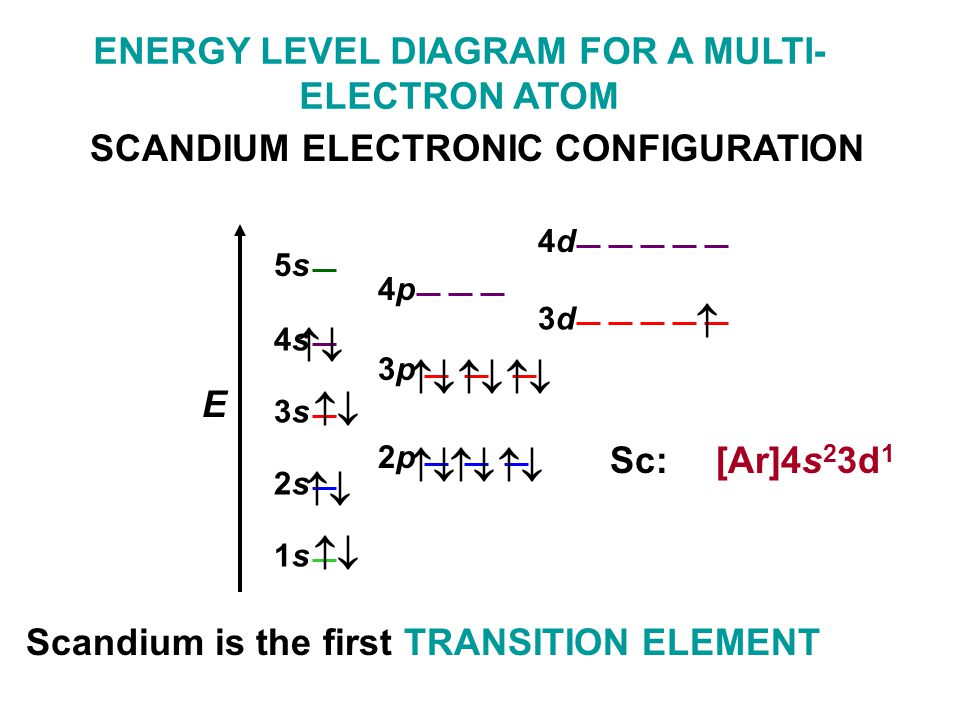
Orbital diagram for scandium. Chemistry questions and answers. Complete an orbital diagram for scandium (Sc). Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Question: Complete an orbital diagram for scandium (Sc). Drag the appropriate labels to their respective targets. Labels can be used once, more than once ... 18.04.2014 · The diagram (not to scale) summarizes the energies of the orbitals up to the 4p level. Figure 1: Electronic energies orbitals. The oddity is the position of the 3d orbitals, which are shown at a slightly higher level than the 4s. This means that the 4s orbital which will fill first, followed by all the 3d orbitals and then the 4p orbitals. Similar confusion occurs at higher levels, with so ... Draw an orbital diagram for scandium Sc? 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^1. What is the orbital diagram for bromine? The orbital doagram for Bromine is 2-8-18-17. The orbital diagram for Bromine ... Scandium is the first of the "d block" elements, so we now need to include a set of five d orbitals in our orbital diagram, with one electron occupying one of these boxes. electron configuration (shells): 2,8,9,2 . electron configuration (sub-shells): 1s 2 2s 2 2p 6 3s 2 3p 6 3d 1 4s 2. condensed electron configuration: [Ar] 3d 1 4s 2. orbital diagram (orbital box diagram) : Pairs of electrons ...
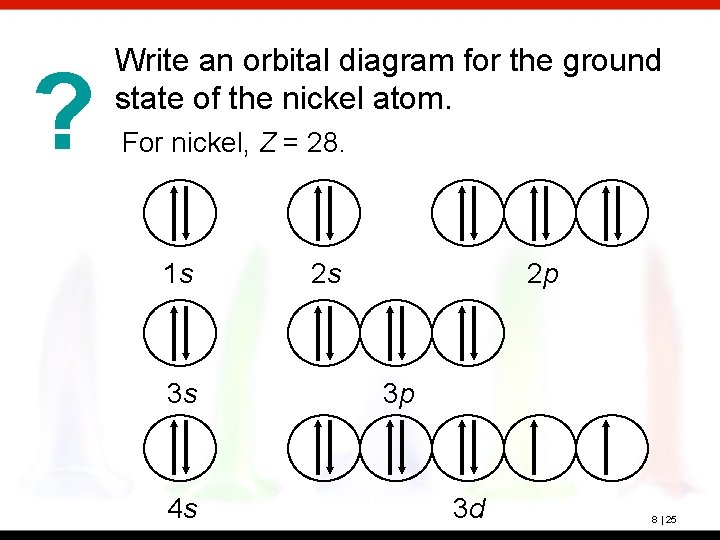
Electron configuration and orbital diagram for scandium ... So for scandium the 1 st and 2 nd electron must be in 1s orbital the 3 rd and 4 th in the 2s the 5 th ... Problem: Part B. Complete an orbital diagram for scandium (Sc)Draw orbital diagrams, and use them to derive electron configurationsTo understand how to draw ... Electron configuration and orbital diagram for scandium The purpose of introducing quantum numbers has been to show that similarities in the electron arrangement or electron configuration lead to the similarities and differences in the properties of elements. But writing the quantum numbers of electrons of an element in set notation like {2,1 ... 29.04.2016 · For Z = 21, scandium, the (n + l) rule says: ... Up to Z = 20 (calcium), the (n + l) rule (and the Aufbau diagram) correctly predicts: Orbital energy levels; The order of occupancy of the orbitals. The physical meaning of the (n + l) rule (and its ability to make these predictions) is related to the size (n) and shape (l) of a given orbital. For Z > 20 (starting at the transition metals): The ...
So the electron configuration of potassium will involve 19 electrons. The full electron configuration of potassium is "1s"^2"2s"^2"2p"^6"3s"^2"3p"^6"4s"^1". The noble gas notation is "[Ar]4s"^1". The following orbital diagram shows the increase in energy from one energy sublevel to the next, but you can write them on the same level horizontally, So here we are going to be drawing the orbital filling diagrams for four different Adams eso. The 1st 1 is our element with the symbol of s or so that is ... The 1s orbital at the bottom of the diagram is the orbital with electrons of lowest energy. The energy increases as we move up to the 2s and then 2p, 3s, and 3p orbitals, showing that the increasing n value has more influence on energy than the increasing l value for small atoms. However, this pattern does not hold for larger atoms. The 3d orbital is higher in energy than the 4s orbital. Such ... For example, compose the electron configuration of scandium, Sc: 1s2 2s2 2p6 3s2 3p6 4s2 3d1 . So because that scandium the 1st and 2nd electron need to be in 1s orbital, the third and 4th in the 2s, the fifth through 10th in the 2p orbitals, etc. You are watching: Complete an orbital diagram for scandium (sc).
Orbital Diagrams. Another way to represent the order of fill for an atom is by using an orbital diagram often referred to as "the little boxes": The boxes are used to represent the orbitals and to show the electrons placed in them. The order of fill is the same but as you can see from above the electrons are placed singly into the boxes before ...
An orbital diagram is similar What is the orbital diagram for. For example, write the electron configuration of scandium, Sc: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 1. So for scandium the 1 st and 2 nd electron must be in 1s orbital, the 3 rd and 4 th in the 2s, the 5 th through 10 th in the 2p orbitals, etc. 6/14/ Ch 8 4/18 Correct Part B Complete ...
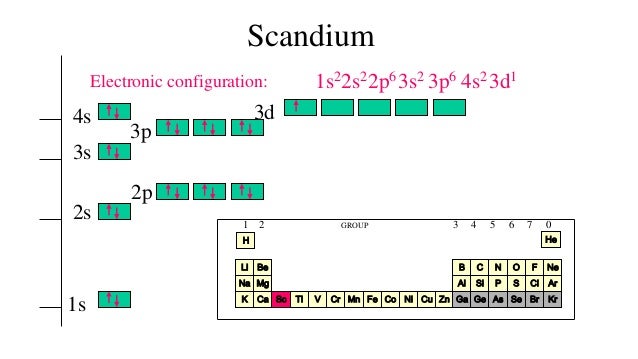
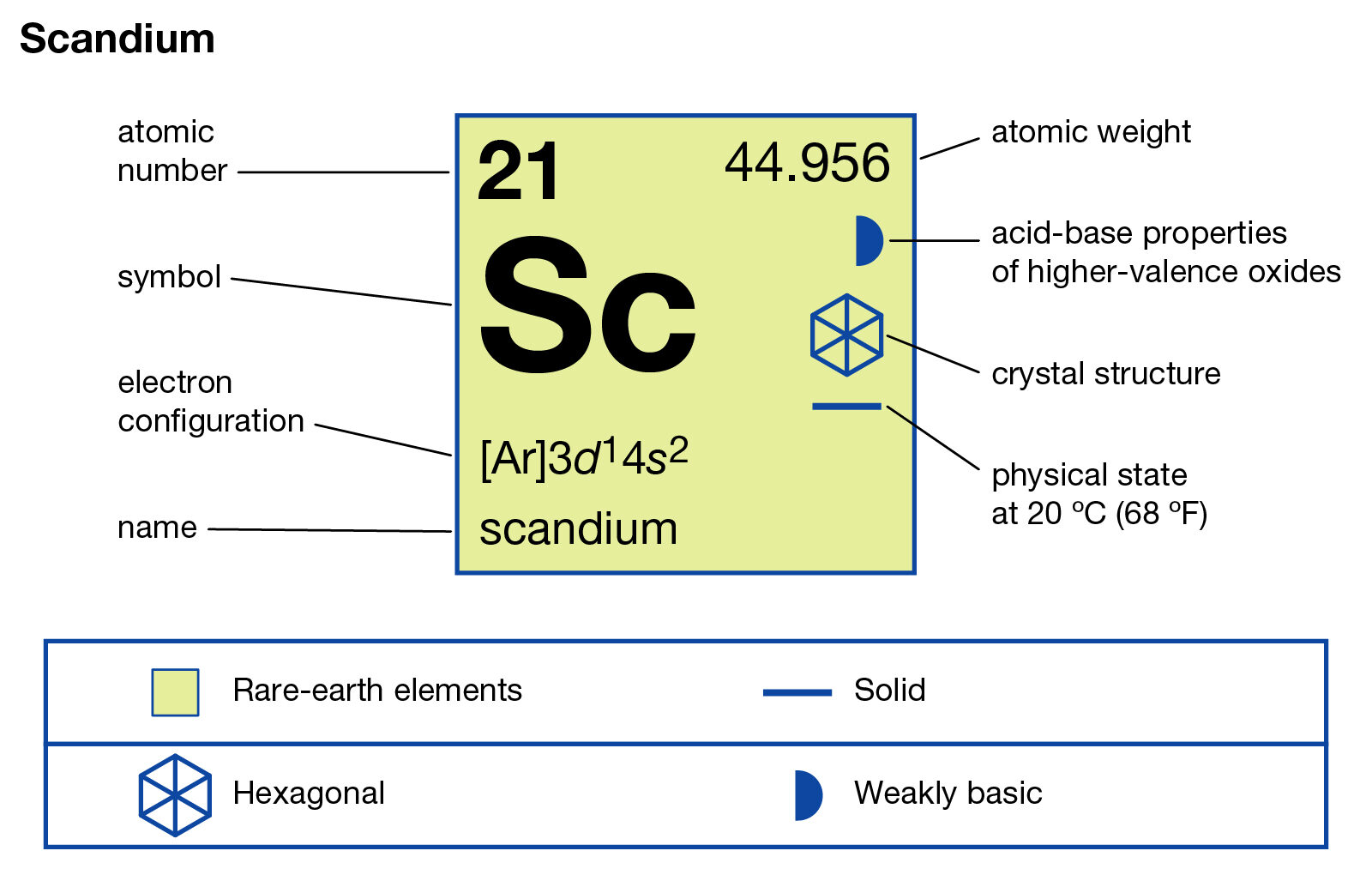
Sc (Scandium) is an element with position number 21 in the periodic table. Located in the IV period. Melting point: 1539 ℃. Density: 2.99 g/cm 3 . Electronic configuration of the Scandium atom in ascending order of orbital energies: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 1. Electronic configuration of the Scandium atom in ascending order of the levels:
Sodium electron configuration is 1s 2 2s 2 2p 6 3s 1.The symbol for sodium is 'Na'. The period of sodium is 3 and it is a s-block element. This article gives an idea about the electron configuration of sodium(Ne) and orbital diagrams, period and groups, valency and valence electrons of sodium, bond formation, compound formation, application of different principles.
Scandium (Sc) has an atomic mass of 21. Find out about its chemical ... Electron Configuration, [Ar] 3d1 4s2. 1s2 2s2 2p6 3s2 3p6 3d1 4s2. Orbital Diagram.
Orbital diagram scandium. Check out the blackboard. That box on the left has all of the information you need to know about one element.
Draw an orbital diagram for scandium {eq}\mathbf {(Sc)} {/eq}. Orbital Diagram: An orbital diagram is the representation of electrons present in different orbitals. There are four orbitals, s ...
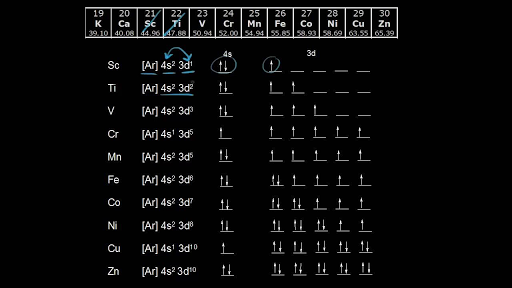
01.11.2021 · Orbital diagram of Scandium (Sc) 22: Orbital diagram of Titanium (Ti) 23: Orbital diagram of Vanadium (V) 24: Orbital diagram of Chromium (Cr) 25: Orbital diagram of Manganese (Mn) 26: Orbital diagram of Iron (Fe) 27: Orbital diagram of Cobalt (Co) 28: Orbital diagram of Nickel (Ni) 29: Orbital diagram of Copper (Cu) 30: Orbital diagram of Zinc (Zn) 31: Orbital diagram of …
1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^1. This compound is made from Scandium and chlorine ions. Scandium is a metal while Chlorine is a non metal.
The orbital diagram for hydrogen can be represented in the following way. ... For scandium we might consider whether the electron goes into the 3d or the 4p. It turns out the energy of the 3d is lower than the 4p so the d sublevel begins to fill with scandium. The electron configuration for scandium is ...
Beginning with the transition metal scandium (atomic number 21), additional electrons are added successively to the 3d subshell. ... orbital diagram: pictorial representation of the electron configuration showing each orbital as a box and each electron as an arrow. valence electrons: electrons in the outermost or valence shell (highest value of n) of a ground-state atom; determine how an ...
Complete an orbital diagram for scandium (Sc). Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Electron configurations are a shorthand form of an orbital diagram, describing which orbitals are occupied for a given element. For example, 1s^2 2s^2 2p^1 is the electron configuration ...
Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics.
Complete An orbital Diagram for Scandium (sc). draw an orbital diagram for scandium sc draw an orbital diagram for scandium sc draw orbital diagram scandium sc how to draw an orbital diagram for scandium draw an orbital diagram for scandium sc answers an orbital diagram is similar to electron configuration except that instead of indicating the atoms by total numbers each orbital is shown with ...
Full electron configuration for Sc can be represented as: [Ar] 1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹ 4s². What is The Electron Configuration of Scandium.
So for scandium the 1 st and 2 nd electron must be in 1s orbital, the 3 rd and 4 th in the 2s, the 5 th through 10 th in the 2p orbitals, etc. This is a memory device to remember the order of orbitals for the first two quantum numbers. Question: Draw an orbital diagram for scandium (Sc). Use this tool to draw the orbital diagram. I need helpp.
When we write the configuration, we'll put all 21 electrons in orbitals around the nucleus of the Scandium atom. In this video we'll use the ...
Using an orbital diagram, determine the number of unpaired electrons in scandium. Scandium: Scandium (Sc) is a metal found in period 3 and group 3 (in the d-block) of the periodic table.
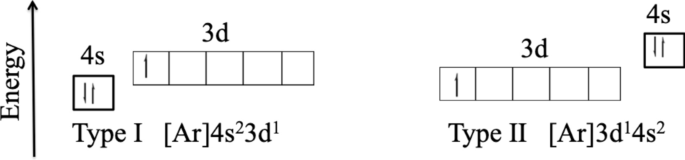
07.11.2013 · According to the aufbau diagram the configuration should be 1s 2, 2s 2, 2p 6, 3s 2, 3p 6, 4s 2, 3d 1 and indeed it is. But conventional wisdom claims that the final electron to enter the atom of scandium is a 3d electron, when experiments indicate that the 3d orbital is filled before the 4s orbital. Why the mistake occurs
Complete an orbital diagram for scandium (Sc). Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Part C Electron configurations are a shorthand form of an orbital diagram, describing which orbitals are occupied for a given element. For example, 1s2 2s2 2p1 is the electron ...
Labels can be used. Answer to Complete an orbital diagram for scandium (Sc). Drag the appropriate labels to their respective targets. Labels can be us.Comprehensive information for the element Scandium - Sc is provided by this page including scores of properties, element names in many languages, most known nuclides and .
Complete an orbital diagram for scandium (Sc). you must draw an electon in boxes diagram the full configuration is: 1s2 2s2 2p6 3s2 3p6 4s2 3d1 s orbital has 1 box (squares) p orbital has 3 boxes d orbital has 5 boxes in each box fill it with arrows (up and down Scandium is a chemical element with the symbol Sc and atomic number 21. For example ...
Use this tool to draw the orbital diagram. Scandium has an atomic no. of Therefore electronic configuration of scandium (Sc) is: 1s2 2s2 2p6 3s2 3p6 3d1 4s2. Here, two electrons are in 4s orbital. For example, write the electron configuration of scandium, Sc: 1s2 2s2 2p6 3s2 3p6 4s2 3d1. So for scandium the 1st and 2nd electron must be.Question ...
Figure 1 depicts the trends of increasing energy with increasing n and increasing l (note that this is different than the orbital diagram that we saw in the previous section for hydrogen because now we have more than one electron). The 1s orbital at the bottom of the diagram is the orbital with electrons of lowest energy. The energy increases as we move up to the 2s and then 2p, 3s, and 3p ...
The above diagram roughly depicts the relative energy difference between these three ways of filling 2 electrons into the three p orbitals. Ground State: 1s22s22p x 12p y 1 (or 1s22s22p x 12p z 1 or 1s22s22p y 12p z 1) [CAUTION: these don’t explicitly state the electron’s spin!] CHEM 2060 Lecture 9: Electronic Configurations L9-3 The 4th Quantum Number In order to discuss atomic orbital ...
Nevertheless check the complete configuration and other interesting facts about Scandium that most people dont know. 614 Ch 8 418 Correct Part B Complete an orbital diagram for scandium. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 1 4s 2 Back to key information about the elementBack to key information about the element. 1s2 2s2 2p6 3s2 3p6 3d1 4s2.
It is perfectly possible that in a neutral scandium atom the 3d sub-level is above the 4s sub-level and that when it ionizes the energy levels ...
The element scandium - one of the so-called transition metals - follows calcium in the periodic table. Scandium has nine (9) electrons in the 3n electron shell. Of these, two are accommodated in the 3s orbital and six more in the 3p orbitals. The ninth electron occupies a d orbital. This is where things start to get really interesting.
The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = +1 2 m s = + 1 2 ).
Complete an orbital diagram for scandium sc. Label all bonds in bf3. Label all bonds in bf3. Label each bond in the molecules as polar or nonpolar and give the shape of each molecule and describe whether each molecule and tell whether each is soluble or insoluble in water. The three sp2 hybrid orbitals have a trigonal planar arrangement to ...
So for scandium the 1st and 2nd electron must be in 1s orbital, the 3rd and 4th in the 2s, the 5th through 10th in the 2p orbitals, etc. You are watching: Enter the orbital diagram for the ion au+. This is a memory device to remember the order of orbitals for the first two quantum numbers. Follow the arrow starting in the upper right, when the ...















0 Response to "37 orbital diagram for scandium"
Post a Comment