37 pi acceptor mo diagram
π Donor vs π Acceptor Ligands The nature of the metal ligand π interaction is dependent on the type of ligand. •π-donor ligands are ligands with one or more lone pairs of electrons in p orbitals on the donor atom that can donate to empty orbitals on the metal. • preferred for metals with high oxidation states and low d electron count ... Feb 15, 2019 · I figured I’d study $\ce{C2O4^2-}$ as an organometallic ligand, and found that it is weak field (pi donor). I hence decided to construct a MO diagram. The oxalate anion is of D2h symmetry, so one can immediately use that, form irreducible representations and find the C-C orbitals:
Hello everyone in this videos we will learn about the type of ligands Pi donor ligands, Pi acceptor ligands, How pi bond is formed means from metal to ligand...

Pi acceptor mo diagram
2. The better the sigma-donating capability (or worse the pi -acceptor ability) of the other ligands on the metal, the lower the CO stretching frequency. 3. For simple carbonyl complexes, counting the number of IR and Raman CO stretching frequencies will often permit one to make a structural assignment. While a sigma bond is always the first bond between two atoms, a pi bond is always the second bond between two atoms (…and third bond, if present). Pi bonds use 2p orbitals to overlap in a bonding and anti-bonding way, generating a pi bonding molecular orbital [ π = (2pa + 2pb)] and a pi-star anti-bonding molecular orbital [ π* = (2pa - 2pb)]. The simplistic mathematics (add the 2p orbitals and • The empty P −R σ*orbital plays the role of acceptor in metal complexes of PR 3. • As the atom attached to the P atom becomes more electronegative, the empty P−X σ*orbital becomes more stable (lower in energy) making it a better acceptor of electron density from the metal centre. 6
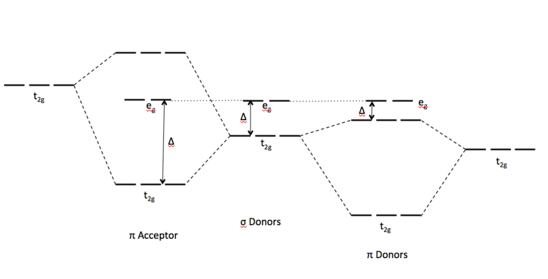
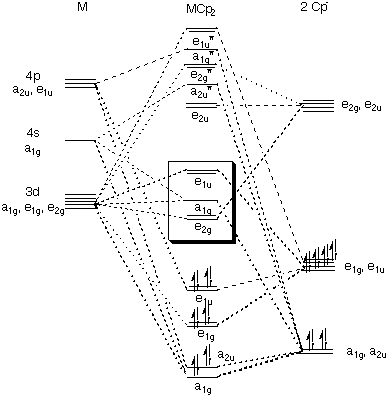
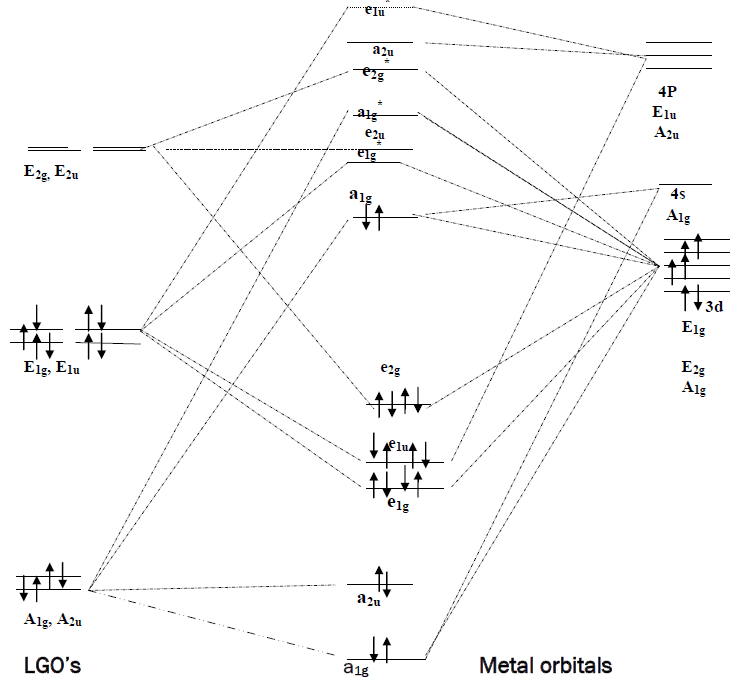
Pi acceptor mo diagram. The MO diagram for generic metallocenes, Cp 2 M is shown below. Notice that the Cp orbitals fill the six lowest orbitals. The next five unoccupied MO's shown in the box have little or no bonding character, which explains our observation above that metallocenes are known for a variety of d-electron counts. MO Diagrams of Pi Donor Ligands and Pi Acceptor Ligands. ... The resulting MO has π* orbitals that are energetically lower than the σ* orbitals that are formed from the non bonding orbitals (e g). The difference between the t 2g π* and e g σ orbitals is denoted as Δ, split. In the π-donor case, the Δ is small due to the low π* level. Pi-Acceptor Ligands Introduction A characteristic feature of the d-block transition metal atoms is their ability to form complexes with a variety of neutral molecules (e.g. carbon monoxide, isocyanides, substituted phosphines, arsines, nitric oxide etc.) and with various molecules with MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H–F nb σ σ* Energy H –13.6 eV 1s F –18.6 eV –40.2 eV 2s 2p So H–F has one σ bond and three lone electron pairs on fluorine
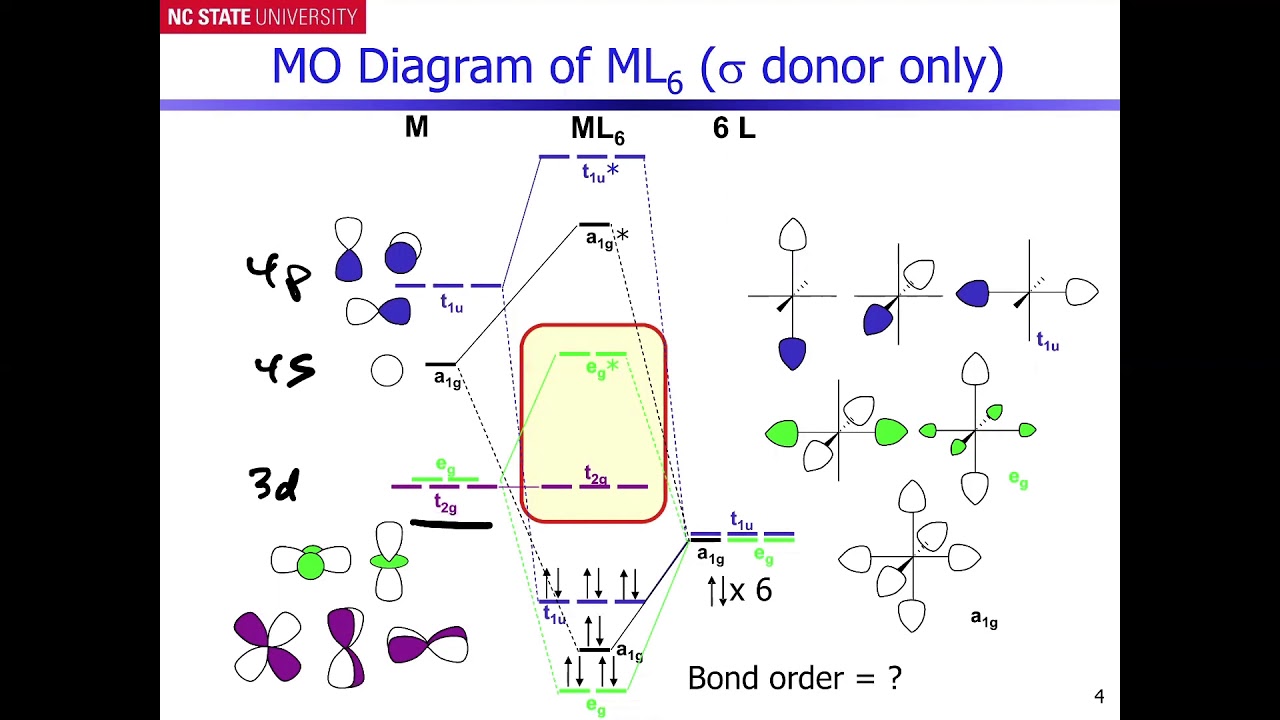
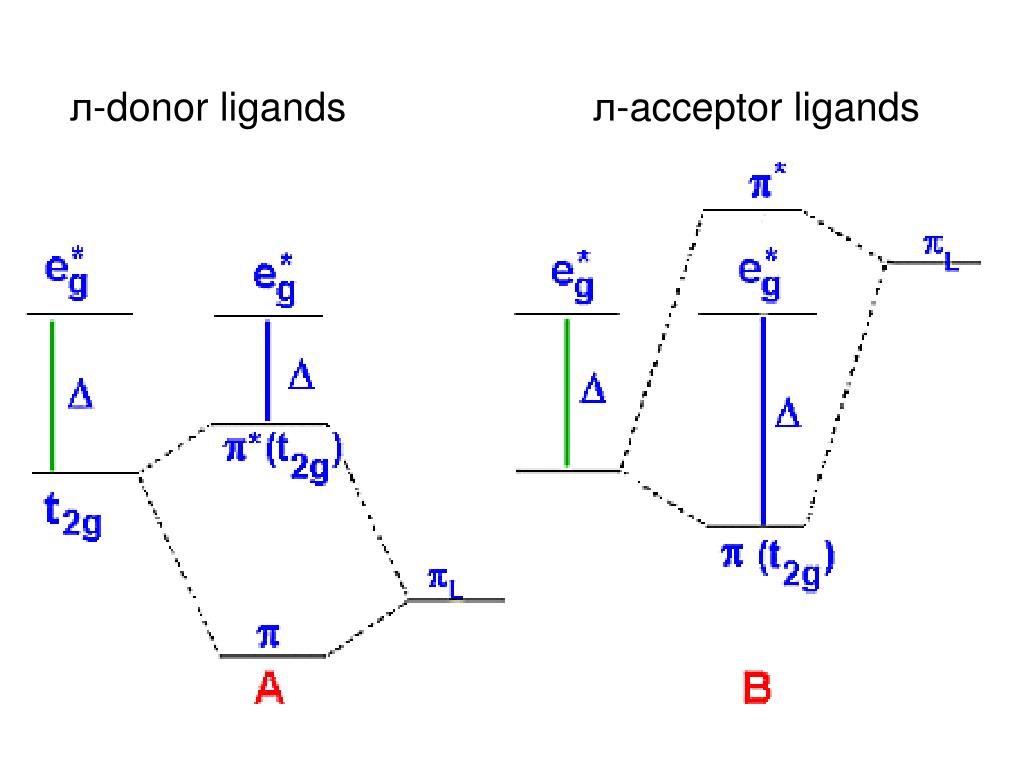
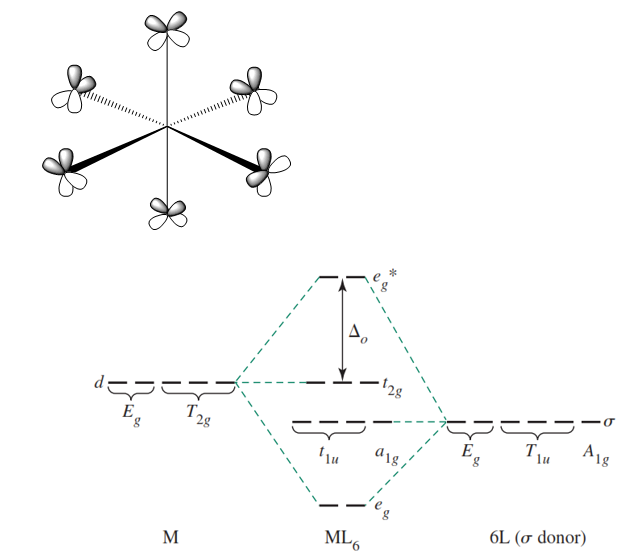
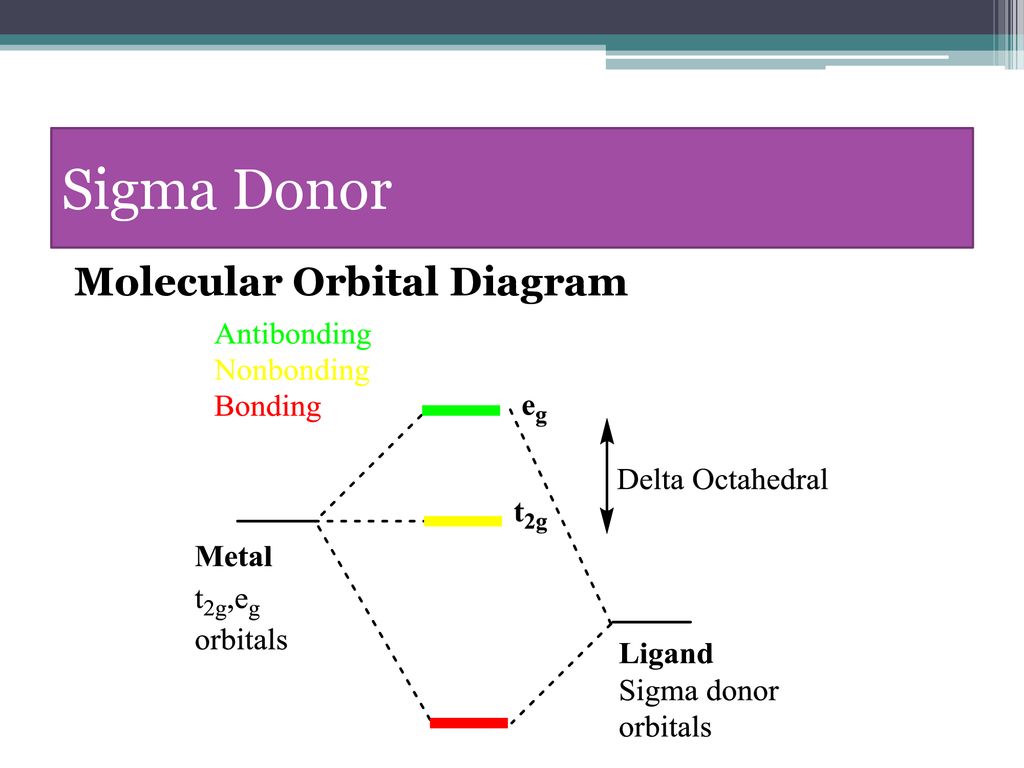
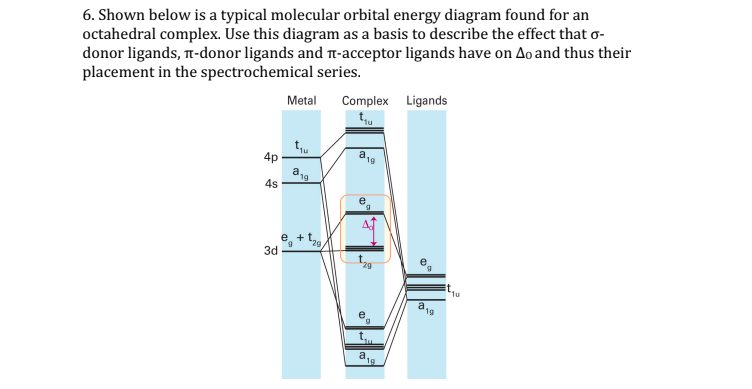
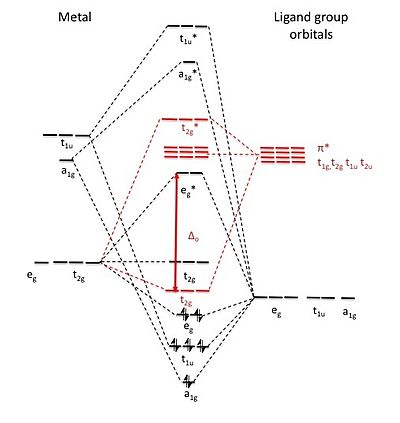
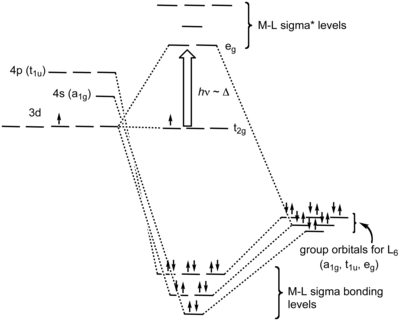
Oct 15, 2020 · Figure 1.11. 1: MO Diagrams of Pi Donor Ligands and Pi Acceptor Ligands. Figure 1.11. 2: Electron configuration of high and low spin. Electron configuration of high and low spin. In a π-donor ligand, the SALCs of the ligands are occupied, hence it donates the electrons to the molecular σ σ* and π π* orbitals. These are the ones that can interact with the p acceptor orbitals. Try adding these to the MO diagram shown above. Again an Rrep can be generated, this time using the pi symmetry orbitals of the ligands; SALC's will have T 1g + T 2g + T 1u + T 2u symmetries. (Try drawing one of the T 2g SALC's.) Molecular Orbital Theory Molecular Orbital Theory Orbital Overlap A bond can only be formed when two atomic orbitals of two atoms overlap Orbitals must be of similar energy To simplify the theory in our context, the 4s and 4p orbitals on the metal atom are ignored. Recall the different types of donor/acceptor behaviour Molecular Orbital Theory – Octahedral, Tetrahedral or Square Planar Complexes The crystal field theory fails to explain many physical properties of the transition metal complexes because it does not consider the interaction between the metal and ligand orbitals. The molecular orbital theory
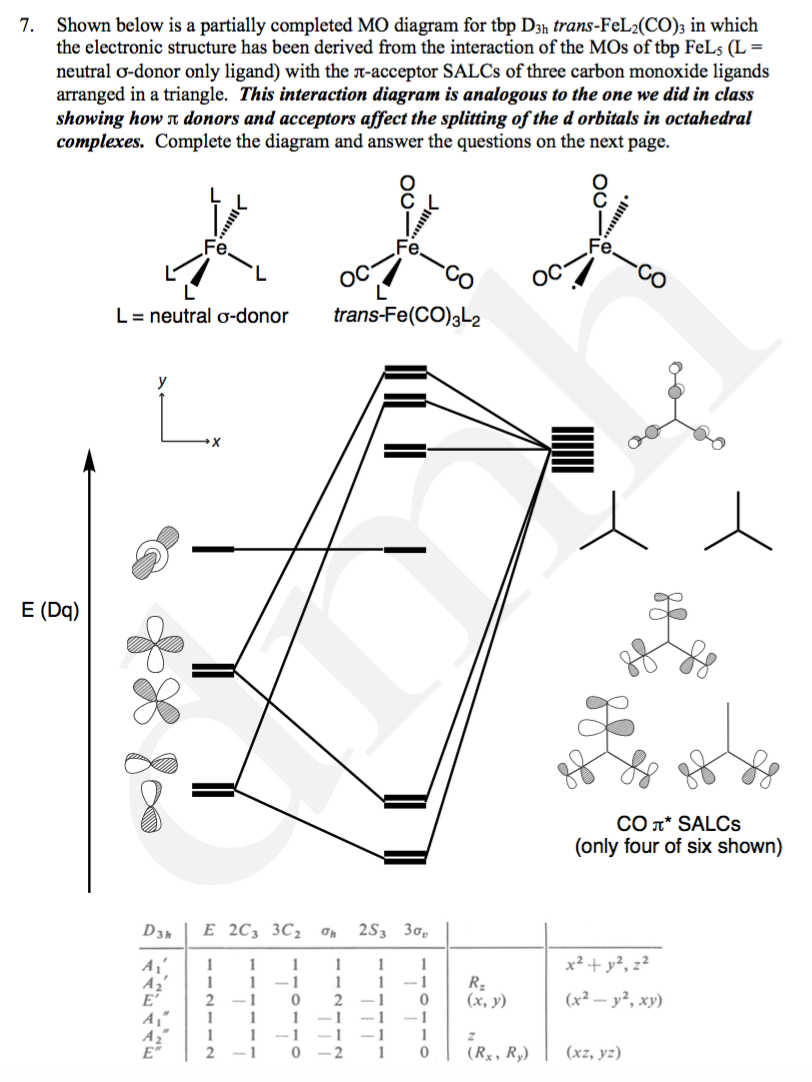
2 Lecture 2 Pi bond (π): bonding molecular orbital –The bonding electron density lies above and below, or in front and in back of the bonding axis, with no electron directly on the bonding axis, since 2p orbitals do not have any electron density at the nucleus. Shown below is a partially completed MO diagram for trigonal bipyramidal D3h trans-FeL 2 (CO) 3 in which the electronic structure has been derived from the interaction of the Molecular orbitals of trigonal bipyramidal FeL 5 (L= neutral sigma donor only ligand) with the pi-acceptor SALCs of three carbon monoxide ligands arranged in a triangle.. Please use D3h irreducible representation chart ... In a UV-visible absorption spectrum, CT transitions appear as relatively intense absorptions with extinction coefficients ( ε) much greater than 1,000. Figure 8.1.2. 1: Abbreviated and annotated MO diagrams for example cases of metal ions in octahdral geometry with sets of π -donor and π -acceptor lignads. Compare the MO diagram of Pt(CN)4 2- and Pt(pyridine)4 2+. Assume the pyridine molecules are all flat in the xy plane. Pyridine is a pi acceptor, but it doesn't have as many pi orbitals as CN- does.
than both, the first antibonding molecular orbital from the σ-bonding (eg*), and the nonbonding sets of t1g, t2u and t1u. On the other hand, the bonding molecular orbitals of t2g are higher in energy than all σ bonding molecular orbitals. The overall molecular orbital energy level diagram for this type of π-bonding in octahedral
π backbonding, also called π backdonation, is a concept from chemistry in which electrons move from an atomic orbital on one atom to an appropriate symmetry antibonding orbital on a π-acceptor ligand. It is especially common in the organometallic chemistry of transition metals with multi-atomic ligands such as carbon monoxide, ethylene or the nitrosonium cation.
The net result is that pi acceptor ligands (such as CO and N 2), with empty antibonding orbitals available to accept electrons from the metal, increase the size of D o. The spectrochemical series can be reconsidered with the possiblity of pi bonding in mind.

How To Rationalise With Mo Theory That Co Is A Two Electron Donor Through Carbon Chemistry Stack Exchange
The sigma bonding MO of H 2 can act as a s donor, while the antibonding H 2 MO acts as a p acceptor (note that the H 2 is side-bound to the metal.) In the case where there is significant backbonding into the H 2 antibonding orbital, the H-H bond will lengthen, up to the point where the bond can break entirely and you would now have two hydride ...
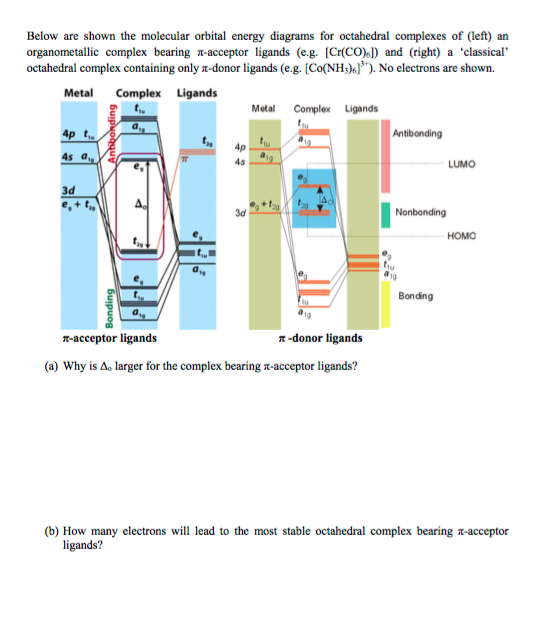
What is the difference between the -donor and -acceptor cases? Below are partial MO diagrams for metal-ligand -bonding in Oh. The orbitals responsible for -bonding are not shown. The energy scale is expanded relative to the -complex MO diagram. Determine which diagram is for the -donor and -acceptor cases.
MO Theory • MO diagrams can be built from group orbitals and central atom orbitals by considering orbital symmetries and energies. • The symmetry of group orbitals is determined by reducing a reducible representation of the orbitals in question. This approach is used only when the group orbitals are not obvious by inspection.
TS Metal-Ligand MOs pi donation pi acceptor. TS Metal-Ligand MOs pi donation pi acceptor.
Answer: Its CO. First of all N2 is a pathetic sigma donor due its non polarity. N2 is infact reduced in its complexes. CO on the other hand has negative charge over carbon atom so it becomes a powerful sigma donor. After donation back bonding occurs between metal atom and anti bonding orbital of ...
The complete orbital interaction diagram is constructed by linear combination of the starting systems, butadiene and ethene (Figure 2.3). The green arrows indicate the two HOMO-LUMO interactions; note that ΔE is small because the HOMO-LUMO energy gap is relatively large (see the relevant equation here).. Figure 2.3 Frontier molecular orbital (FMO) interaction diagram for 1,3-butadiene and ethene.
Jun 10, 2016 · As the LUMO can’t act as a pi acceptor, it’s a sigma donor only. In PR3, the HOMO is again a bonding MO similar to the 3a1 is NH3, BUT the antibonding “e” orbitals involving the p orbitals are lower in energy than the “a1” antibonding. As the LUMO is hence a pi acceptor, it’s strong field.
Pi-Acceptor Interactions The strongest πinteraction is considered to be between a metal d xy orbitals and a ligand π* orbital. Because of the overlap for these orbitals is smaller than the σoverlap, eπ< eσ.
44. π Acceptor Ligands (M L) Ligands such as CN, N2 and CO have empty π antibonding orbitals of the proper symmetry and energy to interact with filled d orbitals on the metal. 45. π Acceptor Ligands (M L) The metal uses the t2g set of orbitals (dxy, dyz and dxz) to engage in pi bonding with the ligand.
Why complexes form. 18-electron rule. Recap of molecular orbital theory. s-donor ligands (hydride complexes). Construction and interpretation of octahedral ML 6 molecular orbital energy diagram Lecture 3: p-acceptor ligands, synergic bonding, CO, CN-, N 2, Lecture 4: Alkenes and alkynes. Dewar-Duncanson-Chatt model. Lecture 5: M(H 2) vs M(H) 2 ...
Actually, it could, but not often. Occasionally it acts as a Lewis acid to stabilize interactions with a transition metal, for instance. CYANIDE COMPARES WELL WITH CARBON MONOXIDE "CN"^(-) is isoelectronic with "CO", and it can act as both a \mathbf(sigma) donor and \mathbf(pi) acceptor. Its MO diagram looks somewhat like that of "CO": We can see the two electrons in the orbital labeled 3sigma ...
• The empty P −R σ*orbital plays the role of acceptor in metal complexes of PR 3. • As the atom attached to the P atom becomes more electronegative, the empty P−X σ*orbital becomes more stable (lower in energy) making it a better acceptor of electron density from the metal centre. 6
While a sigma bond is always the first bond between two atoms, a pi bond is always the second bond between two atoms (…and third bond, if present). Pi bonds use 2p orbitals to overlap in a bonding and anti-bonding way, generating a pi bonding molecular orbital [ π = (2pa + 2pb)] and a pi-star anti-bonding molecular orbital [ π* = (2pa - 2pb)]. The simplistic mathematics (add the 2p orbitals and
2. The better the sigma-donating capability (or worse the pi -acceptor ability) of the other ligands on the metal, the lower the CO stretching frequency. 3. For simple carbonyl complexes, counting the number of IR and Raman CO stretching frequencies will often permit one to make a structural assignment.




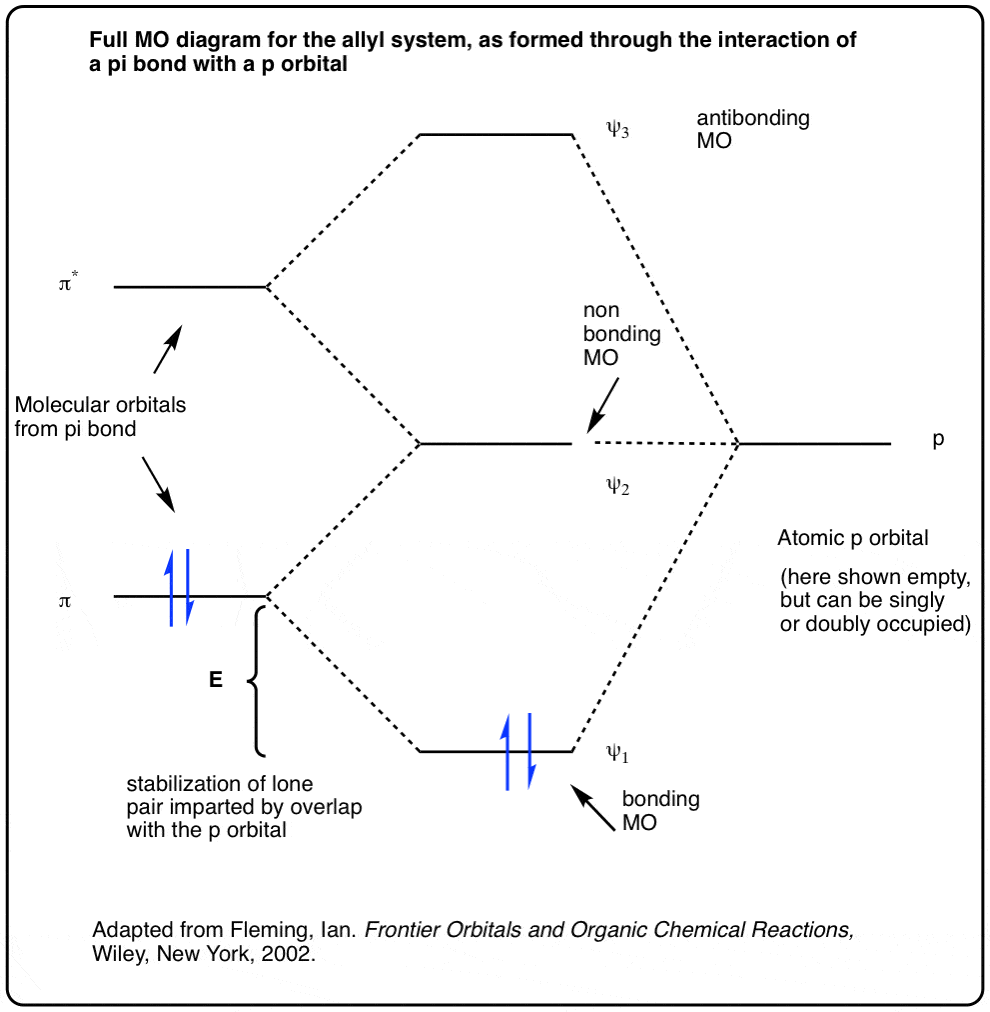
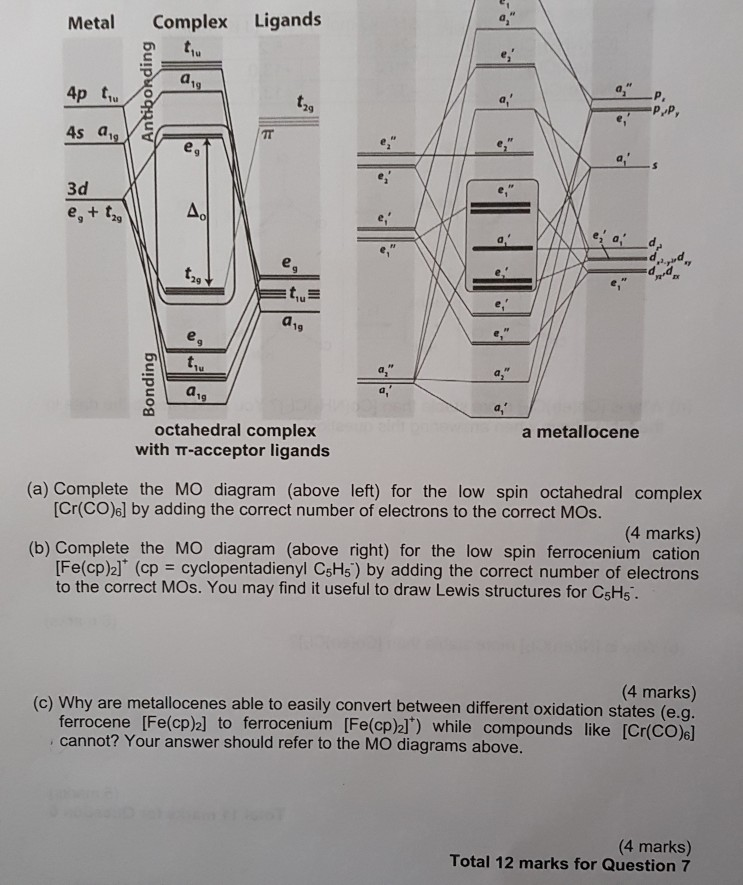
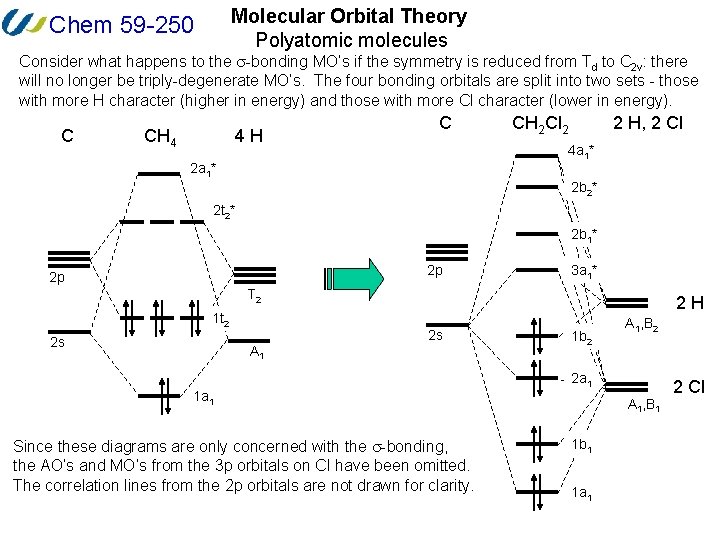








0 Response to "37 pi acceptor mo diagram"
Post a Comment