38 electron distribution diagram of water
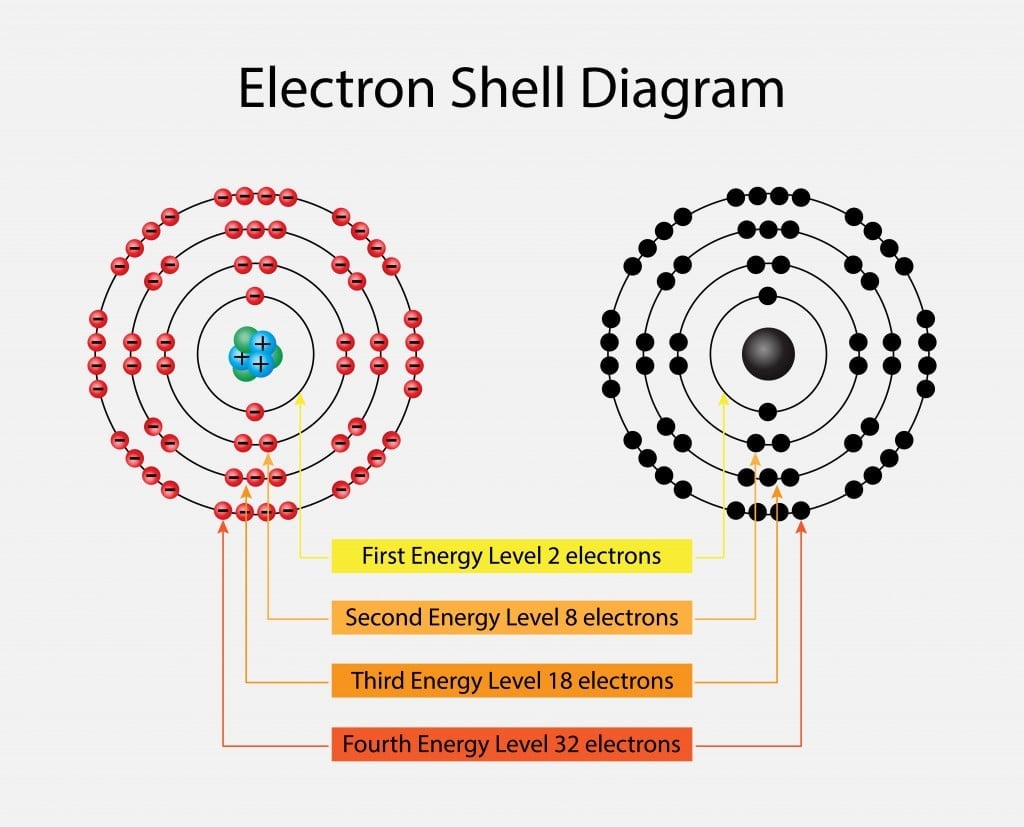
32 Electron Distribution Diagram Of Water. Ditulis oleh Maggie T. Whitehurst Sabtu, 13 Juni 2020 Tambah Komentar. Edit. The negative charge of the electron is balanced by the positive charge of one proton in the hydrogen nucleus. Indicates the number of protons in the nucleus of a specific element as well as the number of electrons in an ... For each electron shell atom diagram, the element symbol is listed in the nucleus. The electron shells are shown, moving outward from the nucleus. The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. The element atomic number and name are listed in the upper left.
Electron distribution diagram of water which element is more electronegative Electrons are shared differently in ionic and covalent bonds. Covalent bonds can be non-polar or polar and react to electrostatic charges. Ionic bonds, like those in table salt (NaCl), are due to electrostatic attractive forces between their positive (Na+) and negative ...

Electron distribution diagram of water
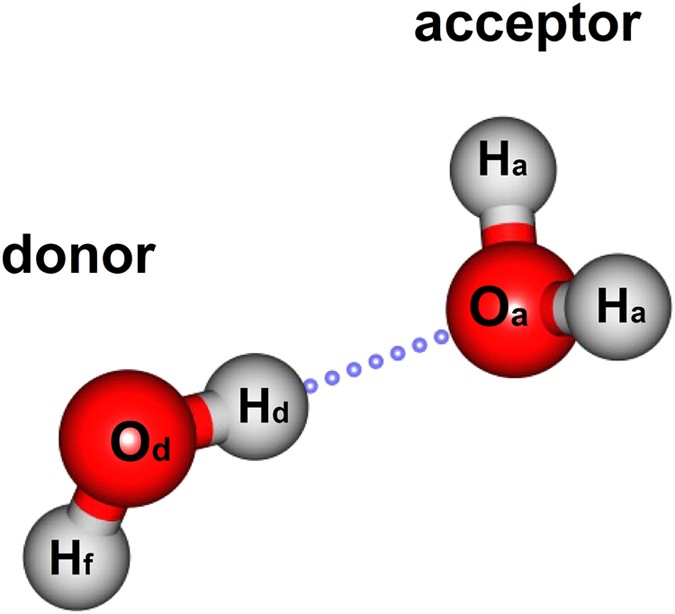
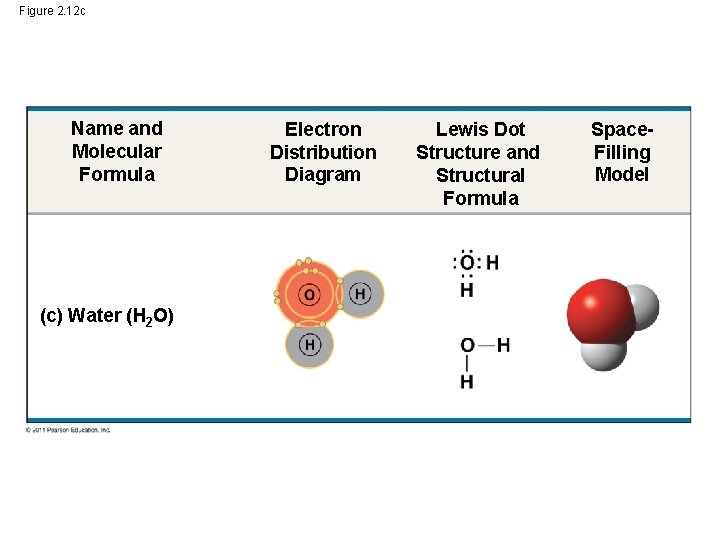
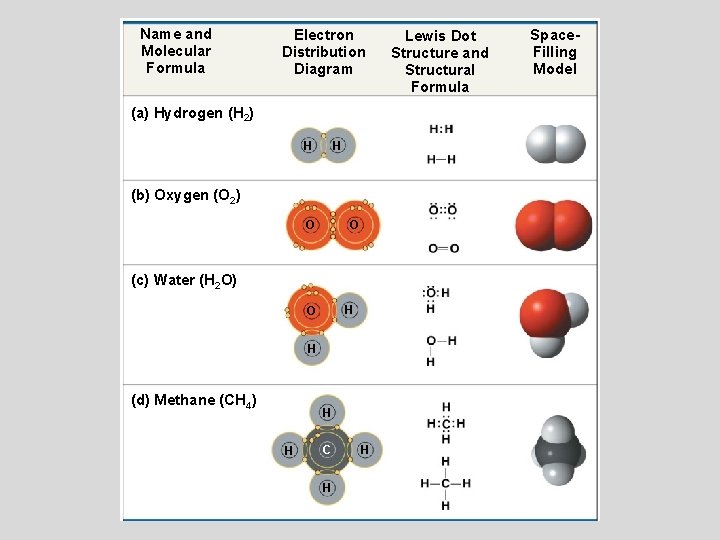
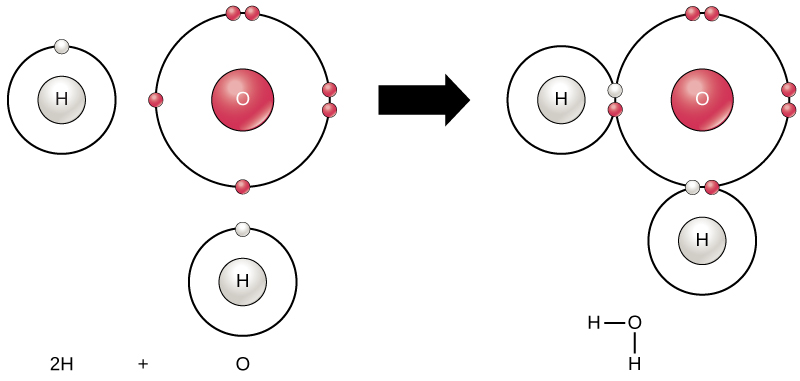
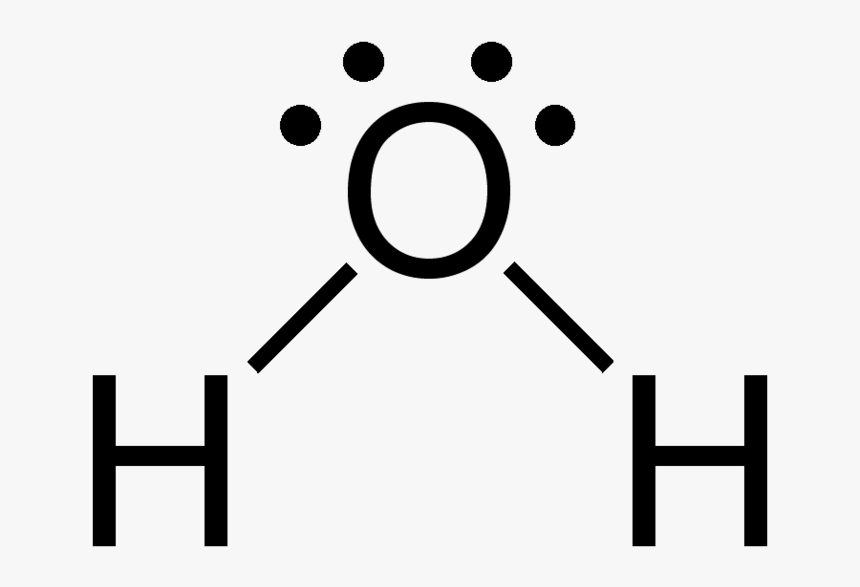
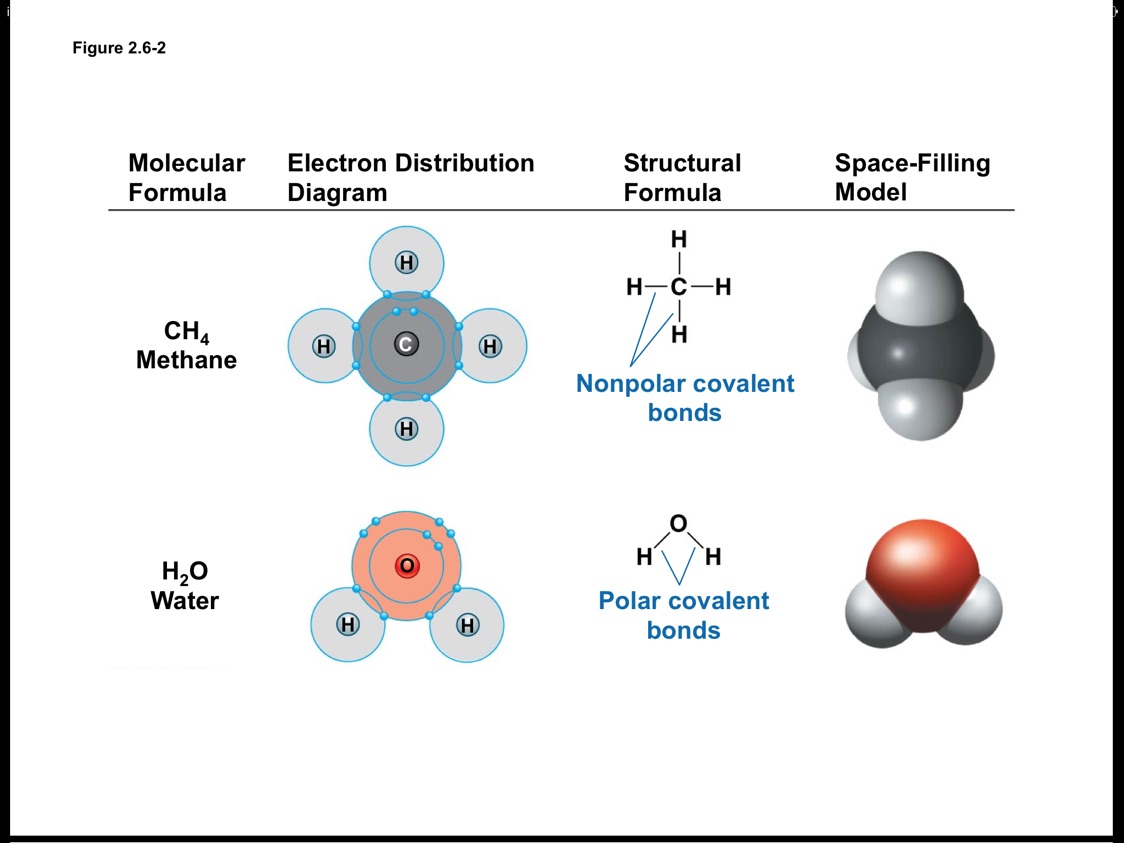
Shape of water molecule Lewis dot diagram O H 104.5o H space filling model. O-H bonds are polarized because of the difference in electronegativity between the O and H atoms. Hydrogen bonds This unequal electron distribution results in strong non-bonding interactions between water molecules - hydrogen bonds. Electron distribution diagram of water The polarity of water Water has a simple molecular structure. It is composed of one oxygen atom and two hydrogen atoms. Each hydrogen atom is covalently bonded to the oxygen via a shared pair of electrons. Oxygen also has two unshared pairs of electrons. Thus there are 4 pairs of electrons surrounding the ... Draw the electron distribution diagram of a water molecule. Water molecule schematic showing full electron shell with charge. So on the water molecule hydrogens are slightly positive less electron density and oxygen is slightly negative higher electron density. Posted on posted on february 11 2019 february 11 2019.
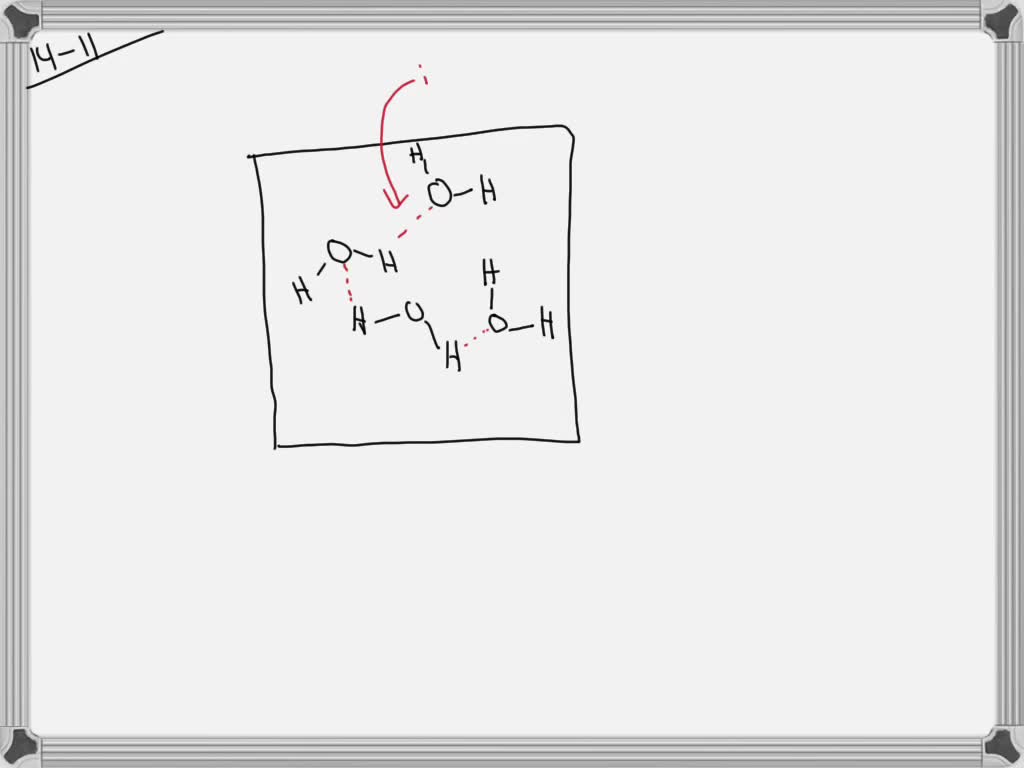
Electron distribution diagram of water. This photo about: Lewis Dot Diagram for Water, entitled as Electron Distribution Diagram Water Lewis Dot Diagram For Water - also describes Electron Distribution Diagram Water and labeled as: lewis dot structure,lewis dot structure practice, with resolution 3808px x 1547px The molecule of water. A molecule is an aggregation of atomic nuclei and electrons that is sufficiently stable to possess observable properties — and there are few molecules that are more stable and difficult to decompose than H 2 O. In water, each hydrogen nucleus is bound to the central oxygen atom by a pair of electrons that are shared between them; chemists call this shared electron pair ... Water is an oxygen hydride consisting of an oxygen atom that is covalently bonded to two hydrogen atoms. It has a role as an amphiprotic solvent, a member of greenhouse gas, a human metabolite, a Saccharomyces cerevisiae metabolite, an Escherichia coli metabolite and a mouse metabolite. 18. Make an electron distribution diagram of water. Which element is most electronegative? Why is water considered a polar molecule? Label the regions that are more positive or more negative. (This is a very important concept. Spend some time with this one!) 19. Another bond type is the ionic bond. Explain what is happening in the figure below ...
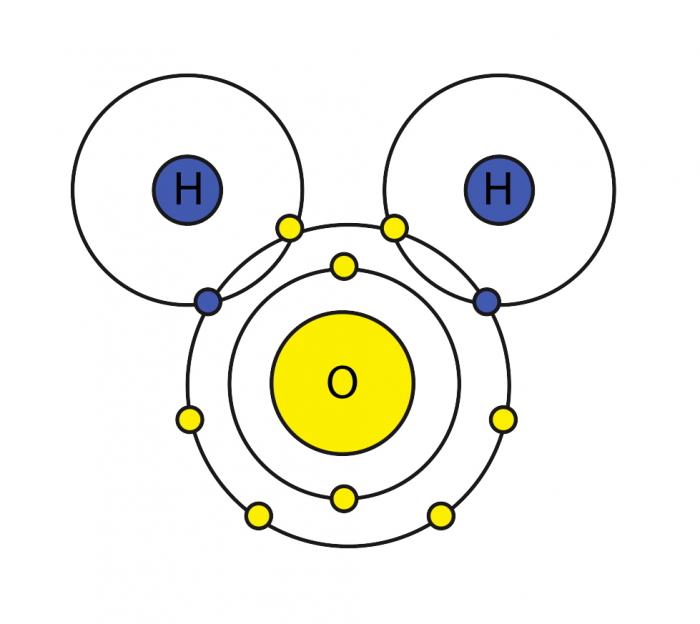
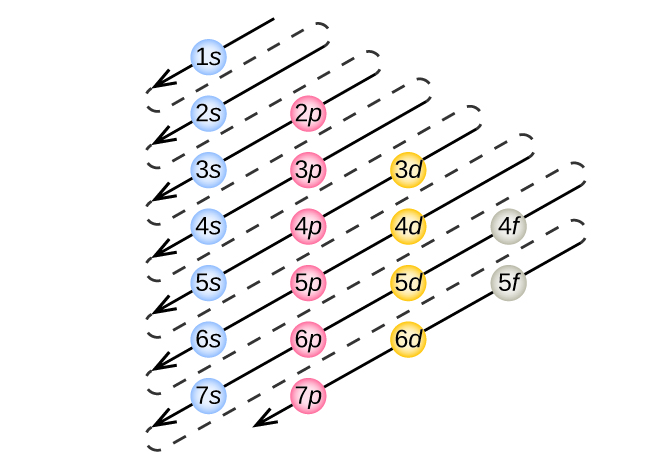
The x-ray structure factor of water measured under ambient conditions with synchrotron radiation is compared with those predicted on the basis of partial structure factors describing the nuclear positions obtained by neutron diffraction and of different assumptions for the electron distribution. The comparison indicates that a charge of approximately 0.5 e is transferred from each hydrogen ... Electron distribution diagram Fig. 2.9: Electrons are distributed in shells of orbitals. Each orbital contains a maximum of two electrons. Chemical behavior of an atom depends mostly on number of electrons in outermost shell called Valence Electrons Water has four core electrons and four valence electrons for its oxygen (1s2, 2s2, 2p4, basically 4 ‘s’-state and 4 ‘p’-state) and its hydrogen atoms share electrons with the oxygen’s outer orbit. Hydrogen’s configuration is ‘1s1’. EDIT: it must follow the rule of eight or the octet rule. The Configuration of the Water Molecule. A molecule of water is composed of two atoms of hydrogen and one atom of oxygen. The one and only electron ring around the nucleus of each hydrogen atom has only one electron. The negative charge of the electron is balanced by the positive charge of one proton in the hydrogen nucleus.
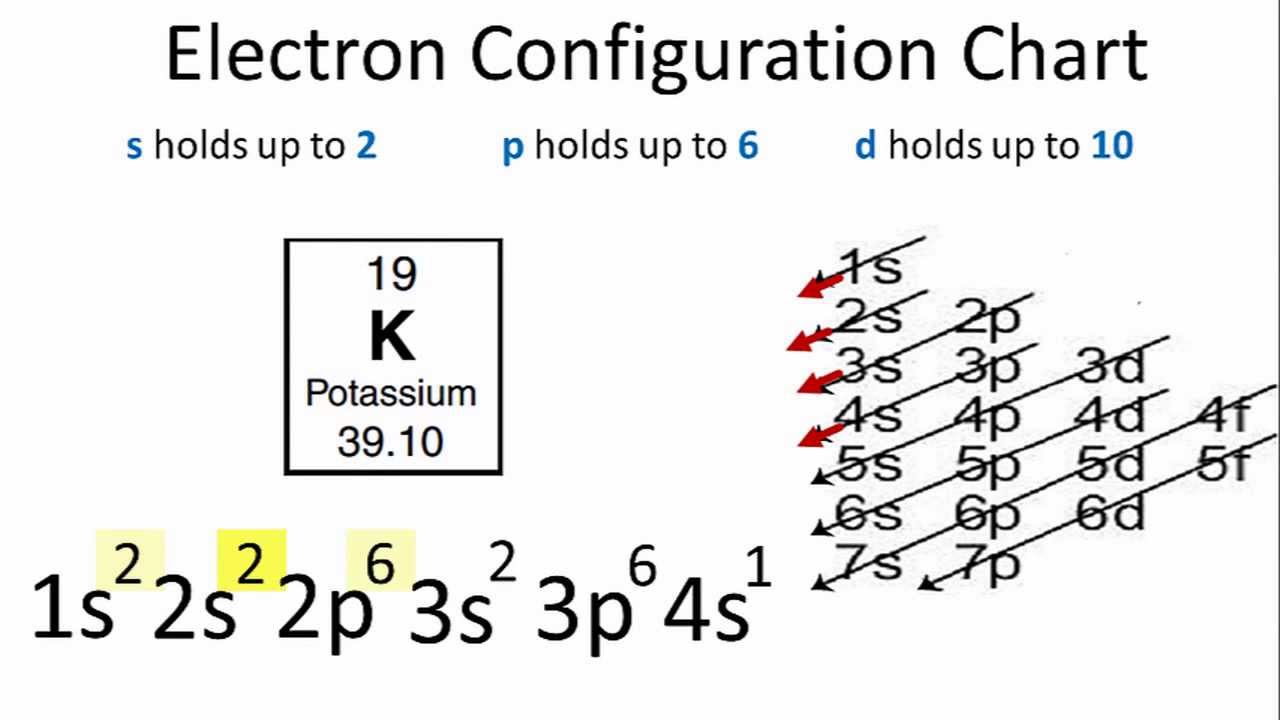
Electron Configuration Chart of All Elements (Full Chart) June 10, 2021 March 7, 2021 by Admin. Electron configuration chart of all Elements is mentioned in the table below. The Shorthand electron configuration (or Noble gas configuration) as well as Full electron configuration is also mentioned in the table. Atomic no. make an electron distribution diagram of water. Subject: Biology Price: Bought 3. Share With. make an electron distribution diagram of water. Which element is most electronegative? Why is water considered a polar molecule? Label the regions that are more positive or more negative Draw the electron distribution diagram for water. Begin with 1 central water molecule. Show the chemistry of each element within the central water molecule (all electron orbits, lone pair electrons, type of chemical bond, polarity/charge, and correct shape). Electron distribution diagram of water. These electron pairs form electron clouds. Indicate the areas with slight negative and positive charges that enable a water molecule to form hydrogen bonds with other polar molecules. Polar covalent bonds occur when one atom is bonded to a more electronegative atom and the electrons of the bond are not ...
Electron Configuration Diagrams | Properties of Matter | Chemistry | FuseSchoolLearn the basics about Drawing electron configuration diagrams. Find out more ...
Jun 01, 2000 · The U.S. Department of Energy's Office of Scientific and Technical Information
The gas phase molecular dipole moment of water has been measured to 1.855 D.6 Assuming the structure of water to be rigid at its experimental geometry, this corresponds to effective atomic charges of q(O) 0.66e and (assuming C 2v symmetry) q(H) 0.33e. Previous studies of the electron density distri-
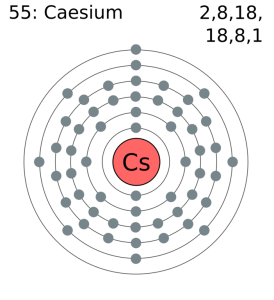
Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element's symbol. A beryllium atom, with two valence electrons, would have the electron dot diagram below. Since electrons repel each other, the dots for a given atom are distributed evenly around the symbol before they are ...
Electron distribution diagram of water. Request pdf on researchgate electron distribution in water the x ray structure factor of water measured under ambient conditions with synchrotron radiation is compared with those predicted on. Label the regions that are more positive or more negative. Electron in the first energy shellelectron in the ...
Make an electron distribution diagram of water. Which element is most electronegative? Why is water considered a polar molecule? Label the regions that are more positive or more negative. (This is a very important concept. Spend some time with this one!)
Feb 13, 2019 · Electron Distribution Diagram Of Water - Hanenhuusholli. Electron angular distribution for 4 MeV electrons in ... Electronic Configuration during the formation of a Water ... Water has many useful properties, and so it is ubiquitous in life on earth. Oxygen atom Why is water considered a polar molecule? because of its shape, it has one side ...
The distribution of electron density in a water molecule is very nearly spherical, and orientational correlation between molecules in the liquid is not "seen" by x rays. Structure and correlation ...
Draw the electron distribution diagram for water. Begin with 1 central water molecule. Show the chemistry of each element within the central water molecule (all electron orbits, lone pair electrons, type of chemical bond, polarity/charge, and correct shape).
Chemistry Q&A Library Draw the electron distribution diagram for water. Begin with 1 central water molecule. Show the chemistry of each element within the central water molecule (all electron orbits, lone pair electrons, type of chemical bond, polarity/charge, and correct shape). What type of bond creates a water molecule?
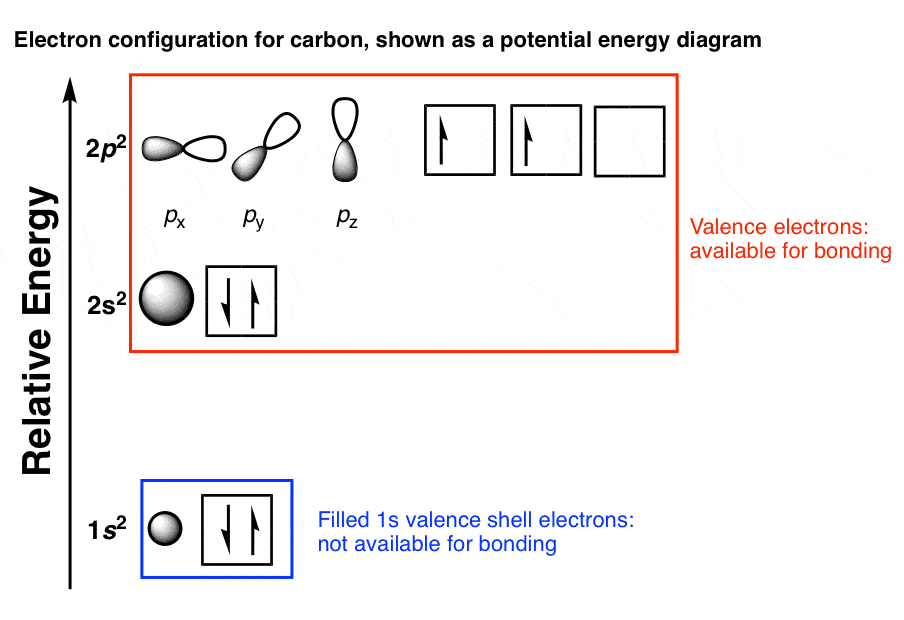
We can illustrate the comparison of orbitals and electron distribution in an isolated boron atom and in the bonded atom in BH 3 as shown in the orbital energy level diagram in Figure 8. We redistribute the three valence electrons of the boron atom in the three sp 2 hybrid orbitals, and each boron electron pairs with a hydrogen electron when B ...
This decades-old question now has an answer, thanks to an article published in Nature Communications on January 16.The study is the result of collaboration among researchers at the University of Chicago, the U.S. Department of Energy's (DOE) Argonne and Lawrence Livermore National Laboratories, and the University of California — San Diego." Knowing the electron affinity of liquid water ...
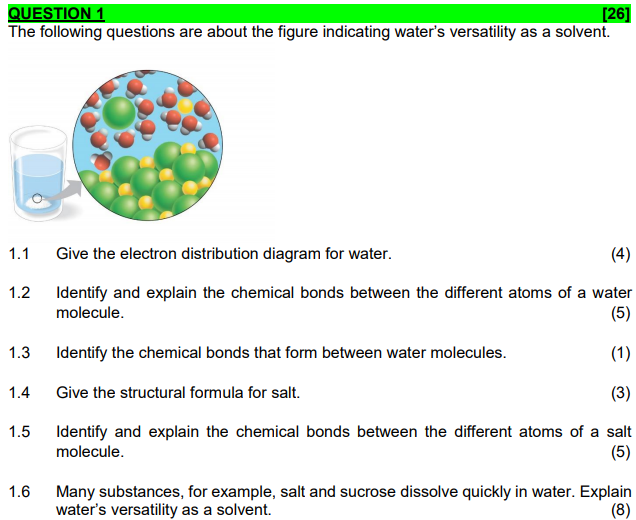
Transcribed image text: QUESTION 1 [26] The following questions are about the figure indicating water's versatility as a solvent. 1.1 (4) Give the electron distribution diagram for water. Identify and explain the chemical bonds between the different atoms of a water molecule. 1.2 (5) 1.3 Identify the chemical bonds that form between water molecules.
Make an electron distribution diagram of water. Which element is most electronegative? Why is water considered a polar molecule? Label the regions that are more positive or more negative. (This is a very important concept. Spend some time with this one!) Diagram on Reading Guide Question 18
Draw the electron distribution diagram of a water molecule. Water molecule schematic showing full electron shell with charge. So on the water molecule hydrogens are slightly positive less electron density and oxygen is slightly negative higher electron density. Posted on posted on february 11 2019 february 11 2019.
Electron distribution diagram of water The polarity of water Water has a simple molecular structure. It is composed of one oxygen atom and two hydrogen atoms. Each hydrogen atom is covalently bonded to the oxygen via a shared pair of electrons. Oxygen also has two unshared pairs of electrons. Thus there are 4 pairs of electrons surrounding the ...
Shape of water molecule Lewis dot diagram O H 104.5o H space filling model. O-H bonds are polarized because of the difference in electronegativity between the O and H atoms. Hydrogen bonds This unequal electron distribution results in strong non-bonding interactions between water molecules - hydrogen bonds.

















0 Response to "38 electron distribution diagram of water"
Post a Comment