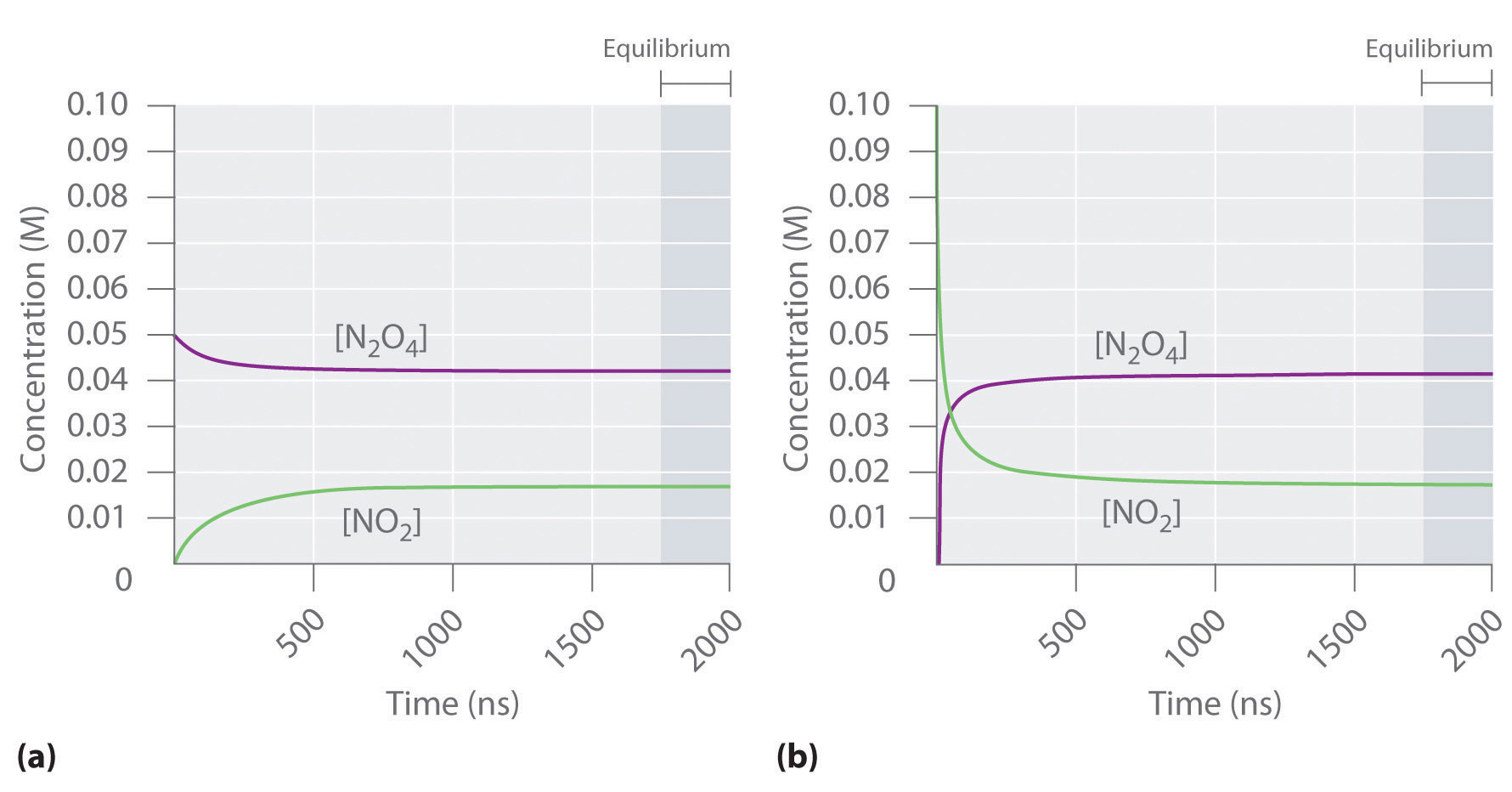
41 in the diagram the black line represents the concentration
20 Questions Show answers. Question 1. SURVEY. 45 seconds. Q. What line segment represents only the solid state? (Diagram F) answer choices. A-B. 1. Draw the tie line. 2. Note where the tie line intersects the liquidus and solidus lines (i.e. where the tie line crosses the phase boundaries). 3. Read off the composition at the boundaries: Liquid is composed of CL amount of Ni (31.5 wt% Ni). Solid is composed of Cαααα amount of Ni (42.5 wt% Ni).
Transcribed image text: In the diagram, the black line represents the concentration of a reactant and the green line represents the concentration Which of the following statements best describes the reaction rate? Time O The reactants maintain an constant concentration in the first half of the reaction O The product maintains an constant concentration in the first half of the reaction At the ...

In the diagram the black line represents the concentration
In the diagram, the black line represents the concentration of a reactant and the green line represents the concentration of a product. Which statement best describes the reaction rate? A. The product maintains an constant concentration in the first half of the reaction. B. The linear relationship between absorbance and concentration displays that absorbance depends on the concentration. Beer’s Law, A=Ebc, helped to develop the linear equation, since absorbance was equal to y, Eb was equal to m, and the concentration, c, was equal to the slope, x, in the equation y=mx+b. To calibrate the spectrometer, a solution ... WILL MARK BRAINLIEST In the diagram, the black line represents the concentration of a reactant and the green line represents the concentration of a product. Which statement best describes the reaction rate? The reactants maintain an constant concentration in the first half of the reaction.
In the diagram the black line represents the concentration. Get an easy, free answer to your question in Top Homework Answers. In the diagram, the black line represents the concentration of a reactant and the green line represents the concentration of a product. Which statement best describes the reaction rate? A. The product maintains an constant concentration in the first half of the reaction. B. At the end of the reaction, both product and reactants ... The graph represents the adult hemoglobin binding curve (in green) at pH 7.4 in the presence of 2,3‑bisphosphoglycerate. ... The mean concentration of hemoglobin in red blood cells is 0.34 g⋅ml−1.0.34 g⋅ml−1. ... no inhibitor- top black line competitive inhibitor - line goes above vmax noncompetitive inhibitor - line does not go above ... In the diagram, the black line represents the concentration of a reactant and the green line represents the concentration of a product. Which statement best describes the reaction rate? A. The product maintains an constant concentration in the first half of the reaction. B. Which statement best describes the reaction rate? A. The product maintains an constant concentration in the first half of the reaction. B. At the end of the ...1 answer · Top answer: Answer:B. At the end of the reaction, both product and reactants are of a constant concentration.Explanation:Option A and C are similar as they both ...
The graph shows the change in concentration of one of the species in the reaction AB→A + B + heat . ... In the diagram, the black line represents the concentration of a reactant and the green line represents the concentration of a product. Which statement best describes the reaction rate? 26 Nov 2019 — answers to question: In the diagram, the black line represents the concentration of a reactant and the green line represents the ...2 answers · 2 votes: B. At the end of the reaction, both product and reactants are of a constant concentration.Explanatio... WILL MARK BRAINLIEST In the diagram, the black line represents the concentration of a reactant and the green line represents the concentration of a product. Which statement best describes the reaction rate? The reactants maintain an constant concentration in the first half of the reaction. The linear relationship between absorbance and concentration displays that absorbance depends on the concentration. Beer’s Law, A=Ebc, helped to develop the linear equation, since absorbance was equal to y, Eb was equal to m, and the concentration, c, was equal to the slope, x, in the equation y=mx+b. To calibrate the spectrometer, a solution ...
In the diagram, the black line represents the concentration of a reactant and the green line represents the concentration of a product. Which statement best describes the reaction rate? A. The product maintains an constant concentration in the first half of the reaction. B.

Determination Of Bulk And Surface Properties Of Liquid Bi Sn Alloys Using An Improved Quasi Lattice Theory

Pdf Transcriptomic Hepatotoxicity Signature Of Chlorpromazine After Short And Long Term Exposure In Primary Human Sandwich Cultures Semantic Scholar
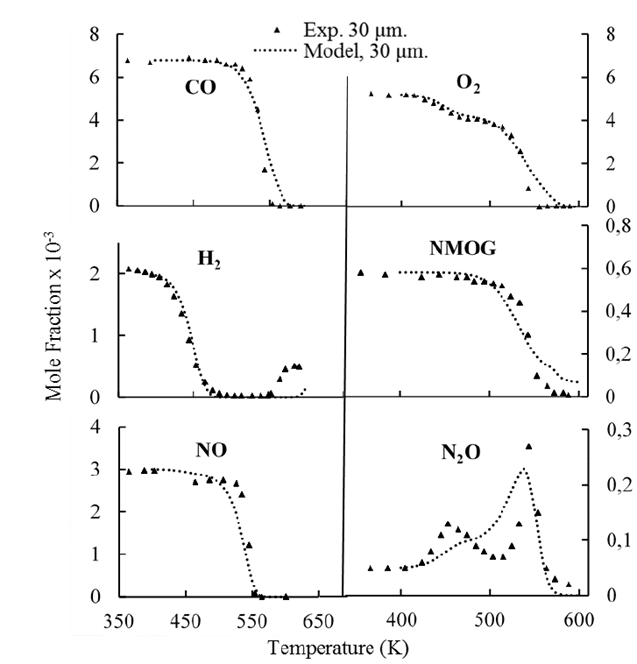
Reaction Kinetic Parameters For A Distributed Model Of Transport And Reaction In Pd Rh Cezro Three Way Catalytic Converters

Extrapolation Of Physiologically Based Pharmacokinetic Model For Tacrolimus From Renal To Liver Transplant Patients Sciencedirect
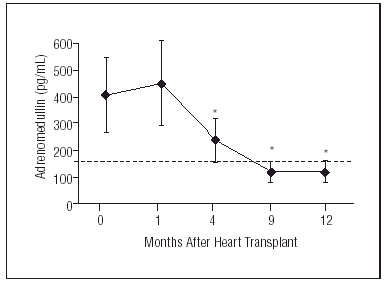
Neurohormonal Activation In Congestive Heart Failure Does It Normalize After Heart Transplantation Revista Espanola De Cardiologia
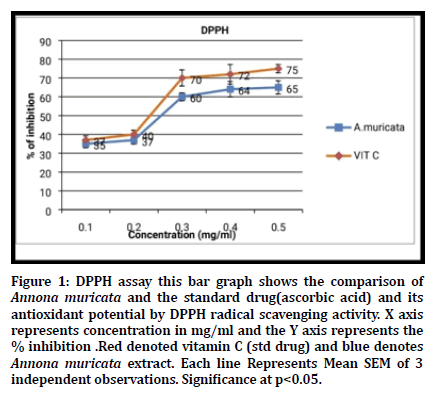
Antioxidant And Antihypercholesterolemic Potential Of Crude Aqueous Leaf Extract Of Annona Muricata An In Vitro Study

Nf Tio2 Photocatalysis Of Amitrole And Atrazine With Addition Of Oxidants Under Simulated Solar Light Emerging Synergies Degradation Intermediates And Reusable Attributes Sciencedirect

Concentration Levels Of Direct Oral Anticoagulants And Clinical Relevance In A Tertiary Hospital Isth Congress Abstracts

Journal Club Review Of Levoketoconazole And Osilodrostat In The Treatment Of Cushing S Syndrome Mgh Neptcc Bulletin
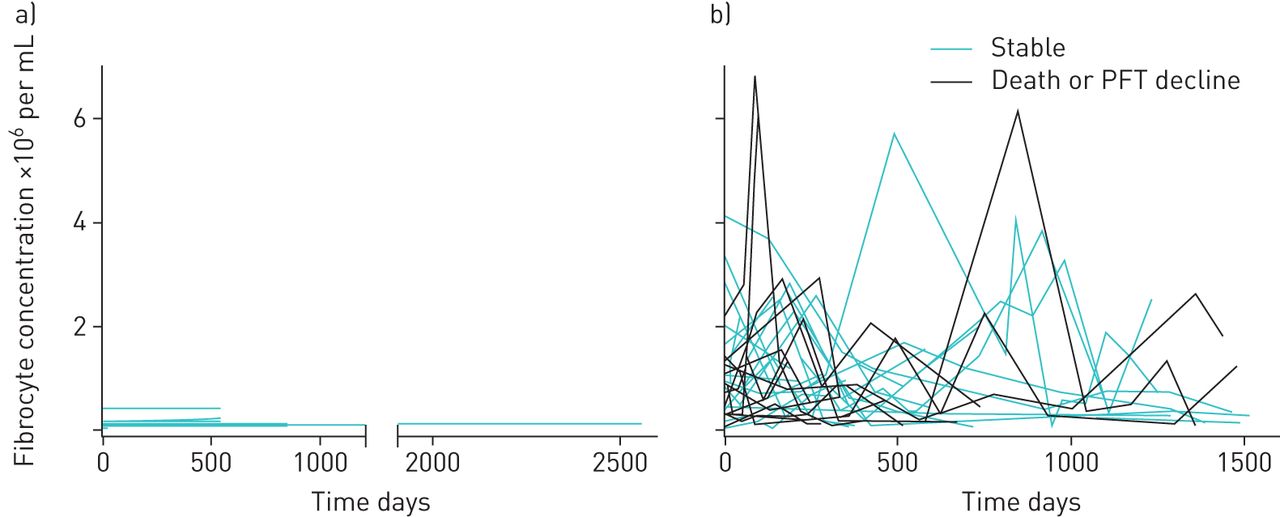
Circulating Fibrocytes As Prognostic Biomarkers Of Autoimmune Interstitial Lung Disease European Respiratory Society
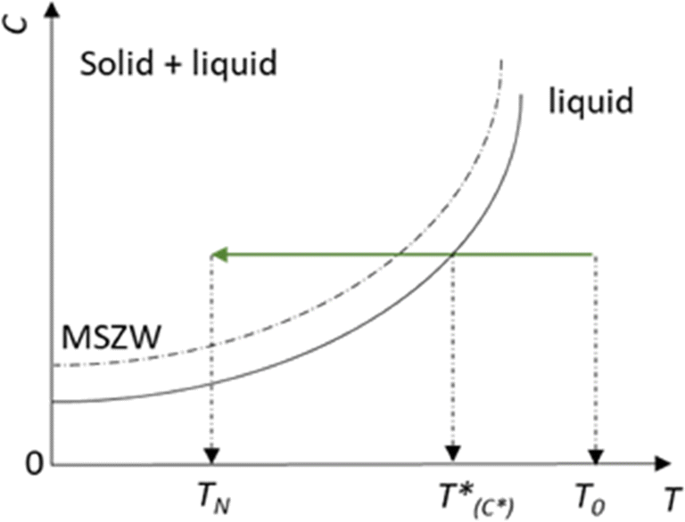
On The Effect Of Flow Restrictions On The Nucleation Behavior Of Molecules In Tubular Flow Nucleators Springerlink

Cerebrospinal Fluid Penetration Of Ceftolozane Tazobactam In Critically Ill Patients With An Indwelling External Ventricular Drain Antimicrobial Agents And Chemotherapy
Groundwater Chemistry And Overpressure Evidences In Cerro Prieto Geothermal Field Document Gale Academic Onefile
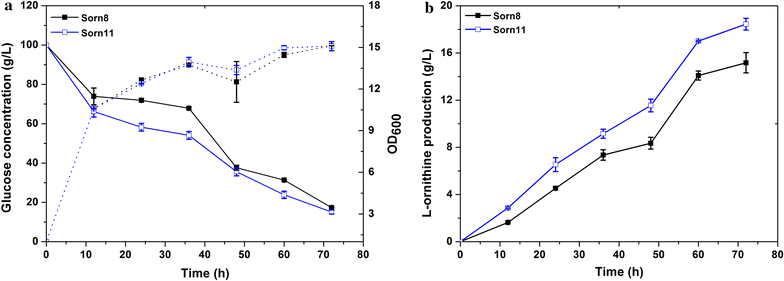
Systematic Pathway Engineering Of Corynebacterium Glutamicum S9114 For L Ornithine Production Microbial Cell Factories Full Text

Plasma Ropivacaine Concentrations During Bilateral Transversus Abdominis Plane Infusions British Journal Of Anaesthesia
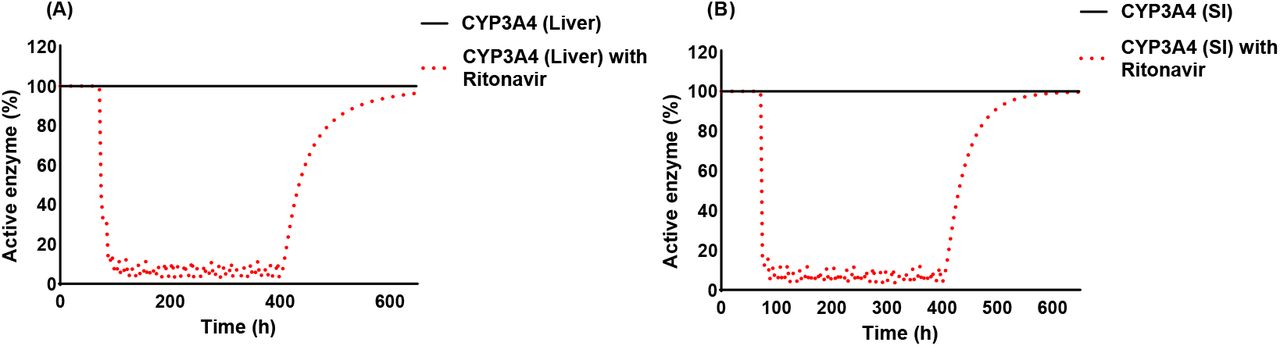
Investigation On The Interaction Between Nifedipine And Ritonavir Containing Antivirus Regimens A Physiologically Based Pharmacokinetic Pharmacodynamic Analysis Medrxiv
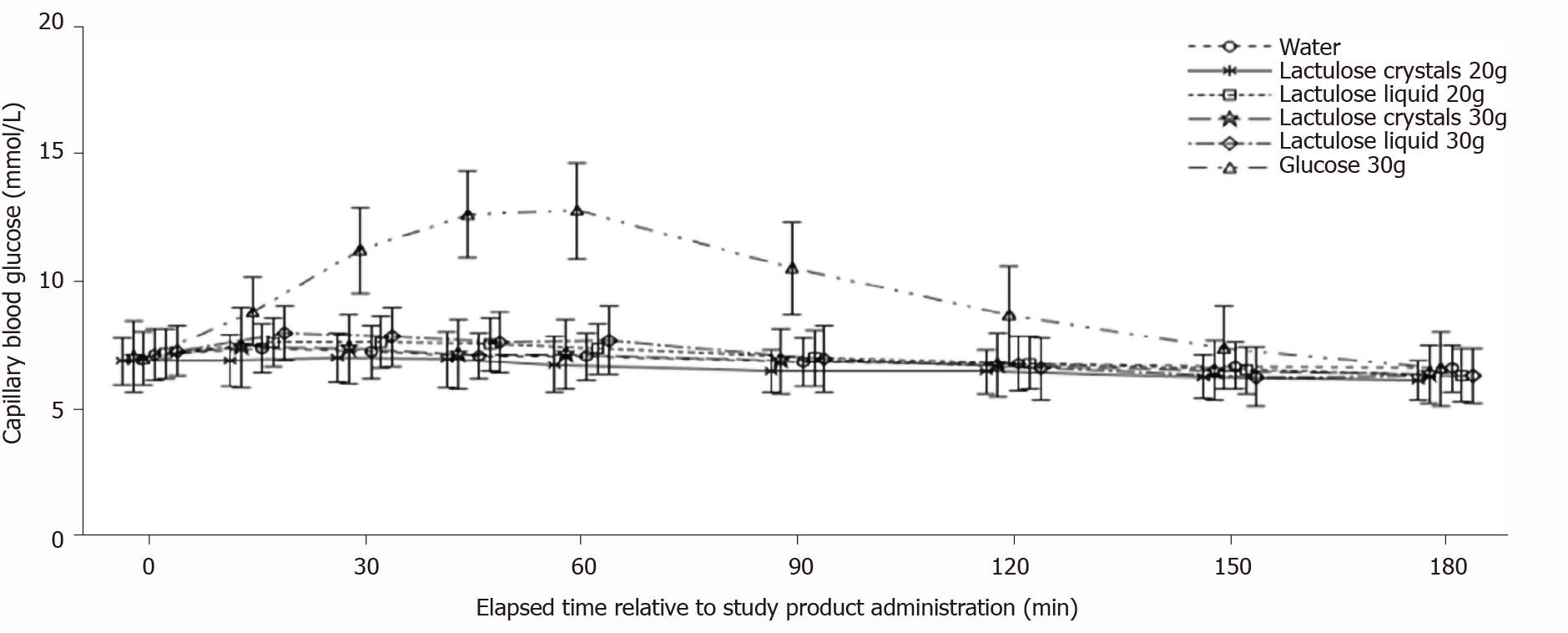









0 Response to "41 in the diagram the black line represents the concentration"
Post a Comment