41 match the appropriate octahedral crystal-field splitting diagram fe4+
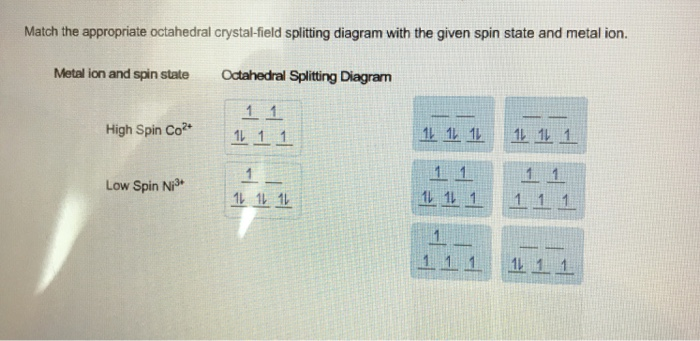
Answer to Draw the crystal field splitting diagram for each of the following complex ions. Fe(CN)6 4- Ni(CN)4 - FeCl6 3- Mn(H2O)6 ... Question: Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. High Spin Fe^4+ Low Spin Mn^3+ ...
Consider the complex ion [Mn (H2O)6]2+. Draw the crystal field energy level diagram and use arrows (that point up or down) to show the placement of d electrons assuming that this is (a) a weak field complex ion (b) stron field complex ion. Our mission is to help you succeed in your Chemistry class.
Match the appropriate octahedral crystal-field splitting diagram fe4+
In the model of the crystal field with parameters 10Dq=9500 cm −1 , trigonal splitting C=500 cm −1 , the electronic transition to A g -5 E g at a wavelength of 1.66 μm in the tetrahedron in ... CRYSTAL-FIELD SPLITTING DIAGRAMS All four of these transition metals commonly have coordination numbers of \mathbf(6), however, so let's examine their octahedral complex crystal-field splitting diagrams. HIGH SPIN VS. LOW SPIN High spin = fill all five d orbitals with one electron first, and then double up. Low spin = fill lowest-energy d ... Sharing MIT's Tradition of Excellence, we commit to changing the world through research, education, and community efforts · 77 Massachusetts Ave., 18-393 | Cambridge, MA 02139 | 617-253-1803 For Emergencies | Accessibility © 2018 MIT Department of Chemistry
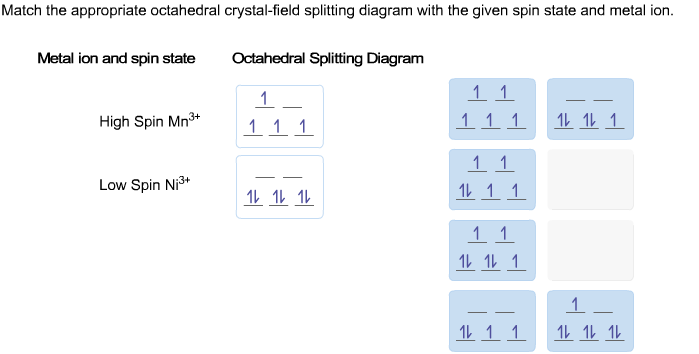
Match the appropriate octahedral crystal-field splitting diagram fe4+. The d-orbital splitting diagram is the inverse of that for an octahedral complex. 6. 7. Page 7 of 33 Crystal Field Splitting Parameters In an octahedral or a tetrahedral crystal field, the d-orbitals are split into two sets. The energy separation between them is called the crystal field splitting parameter. This section contains the course materials for Unit IV, including lecture videos, readings, lecture notes, and practice problems. The crystal field stabilization ... ion in the crystal field generated by a set of ligands. It arises due to the fact that when the d orbitals are split in a ligand field, some of them become lower in energy than before. For example, in the case of an octahedron, the t2g set ... Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. Metal ion and spin state Octahedral splitting diagram Answer Bank high-spin Mn2+ 1 1 1 1 1 1 1 1 1. 11 11 11 low-spin Mn2+ 1L 1L 1 1 1 1 1 1 1 11 1 1 1 1 1
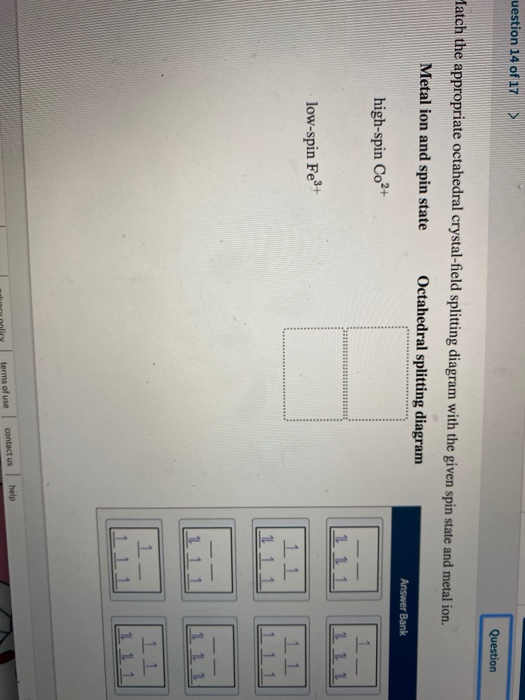
Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. High-spin Mn^3+ Low-spin Fe^3+. Sign up to view answer. Our mission is to help you succeed in your Chemistry class. Sign up for free to see the solution. Match the appropriate octahedral crystal-field splitting diagram with the ... ion and spin state Octahedral splitting diagram Answer Bank high-spin Fe4+ 11 ... Electrons in Orbitals. According to the Aufbau principle, electrons are filled from lower to higher energy orbitals (Figure \(\PageIndex{1}\)).For the octahedral case above, this corresponds to the d xy, d xz, and d yz orbitals. Following Hund's rule, electrons are filled in order to have the highest number of unpaired electrons.For example, if one had a d 3 complex, there would be three ... August 11, 2020 - https://chem.libretexts.org/@a...norganic_Chemistry/Readings/Week_4%3A_Ligand_Field_Theory_(Octahedral_Complexes)/4.1%3A_Ligand_Field_Theory_(LFT)_and_Crystal_Field_Theory_(CFT)_of_Octahedral_Complexes/4.1.2%3A_Introduction_to_Ligand_Field_Theory_(Octahedral_comple...
Transcribed image text: Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. Metal ion and spin state Octahedral Splitting Diagram High Spin Fe^3+ Low Spin Co^2+ Answer + 20 Transcribed image text: Question 17 of 25 > Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. Metal ion and spin state Octahedral splitting diagram IIIIIIIIIIIIIIIIIIIIIIIII Answer Bank high-spin Fe4+ low-spin Mn3+ 11 1 1 1 11 1 1. This problem has been ...
Get the detailed answer: Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. High Spin Mn^3 + Low Spi
Match the appropriate octahedral crystal field splitting diagram. As a result the splitting observed in a tetrahedral crystal field is the opposite of the splitting in an octahedral complex. The octahedral ion feno 2 6 3 which has 5 d electrons would have the octahedral splitting diagram shown at right with all five electrons in the t 2g level.
Crystal field splitting diagram posted on february 10 2017 by admin crystal field theory chemistry 2 pinterest and crystal field splittings in various geometries image for match the appropriate octahedral crystal field splitting diagram with given spin state image of page 3.
Central Tenants of Crystal Field Theory • The metals (Lewis acids) have d orbitals that are partially filled with electrons. • Ligands, that are Lewis bases with lone pairs, come in and form a covalent bond.
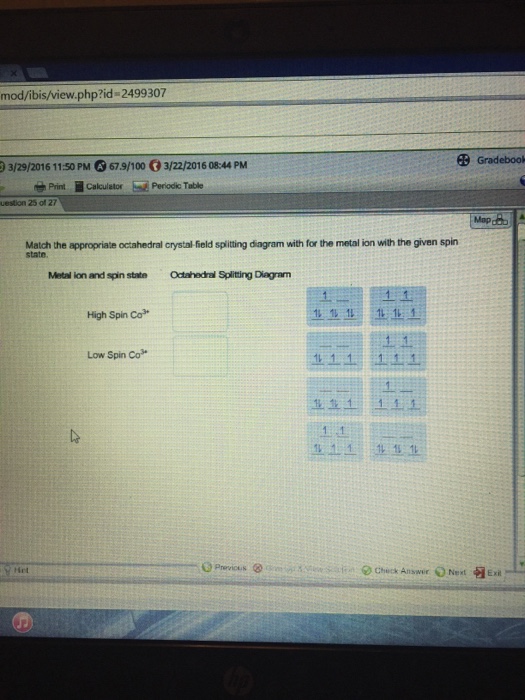
Show transcribed likeness text Match the embezzle octahedral crystal-field splitting diagram with the abandoned alter specify and metal ion. Metal ion and alter specify Xdahedral Splitting Diagram High Alter Co3* Low Alter Fe* 1L 1 1 Match the embezzle octahedral crystal-field splitting diagram with the abandoned alter specify and metal ion. Metal ion and alter […]
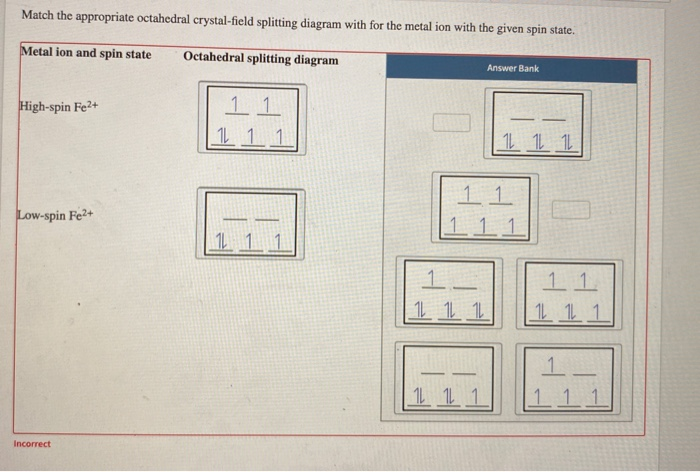
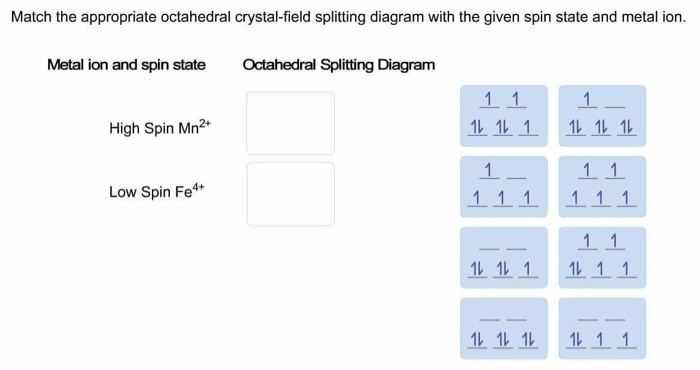
Match the appropriate octahedral crystal-field splitting diagram with the ... diagram Answer Bank high-spin Fe++ 1 1 1 1 1 1 1 1 1 low-spin Fe4+ 1 1 1 1 1 1 ...
Metal ion and spin state Octahedral splitting diagram Answer Bank high-spin Mn3+ low-spin Fe4+ 2 12. This problem has been solved! See the answer ...
This chemistry video tutorial provides a basic introduction into crystal field theory. It explains how to draw the crystal field splitting diagram of transit...
Match the appropriate octahedral crystal field splitting diagram fe4. This theory has been used to describe various spectroscopies of transition metal coordination complexes in particular optical spectra colors. Cobalt 3 has 6 d electrons cobalt normally has 9 valence electrons but youve lost 3. Crystal field stabilization energies for ...
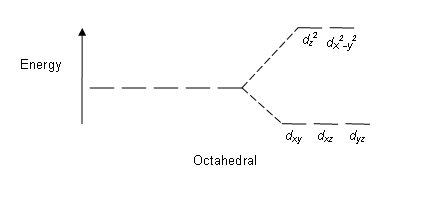
Crystal Field Splitting in an Octahedral Field eg Energy 3/5 o o 2/5 o t2g e g - The higher energy set of orbitals (d z2 and d x2-y2) t 2g - The lower energy set of orbitals (d xy, d yz and d xz) Δ o or 10 Dq - The energy separation between the two levels The eThe eg orbitals are repelled by an amount of 0 6orbitals are repelled by an amount of 0.6 Δo The t2gorbitals to be stabilized to the ...
Answer to: Match the appropriate octahedral crystal-field splitting diagram with the given spin stale and metal ion with given spin state. [{Image...
Construct the octahedral crystal field splitting diagram for the metal in each species. Since the oxalate ligand is fairly low in the series a weak field ligand at this point you may not have studied ligand field theory yet which explains why it is a weak ligand. Cr4 mnh2o62 asked by katie on march 30 2012 chemistry based on crystal field ...
octahedral complexes, it is also typically high spin and also has 3 unpaired electrons; in square planar complexes, it has 1 unpaired electron. The magnetic moments can be calculated as n(n 2) 3.9, 3.9, and 1.7 B, respectivel y. 10.9 For the red compounds (Me and Et at high temperatures, Pr, pip, and pyr at all temperatures), the
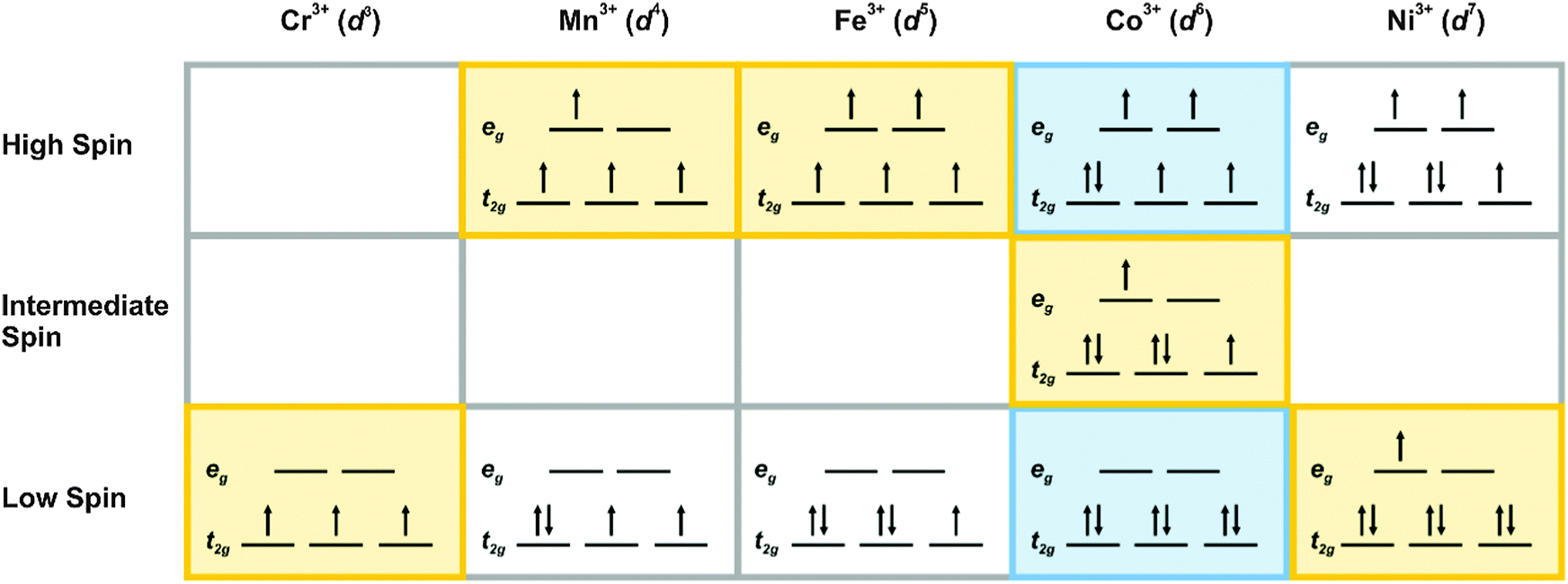
With 4 to 7 d electrons, two options are possible for octahedral complexes. An example is Co3+ in an octahedral complex: Hexafluorocobaltate(III), [CoF6]3-, is characterized by a smaller crystal field splitting (the energy difference between the two sets of d orbitals).
Fig.9.8: d orbital splitting in an octahedral crystal field The crystal field splitting, Δ↓o, depends upon the field produced by the ligand and charge on the metal ion. Some ligands are able to produce strong fields in which case, the splitting will be large whereas others produce weak fields and consequently result in small splitting of d ...
Match the appropriate octahedral crystal field splitting diagram with the given spin state and metal ion. The splitting diagram for square planar complexes is more complex than for octahedral and tetrahedral complexes and is shown below with the relative energies of each orbital. As a result the splitting observed in a tetrahedral crystal field ...
Match the appropriate octahedral crystal field splitting diagram. A none of the 3d orbitals point directly at ligands b t2 orbitals are more stable than e orbitals c a small crystal field splitting energy results in a paramagnetic complex d the low spin case gives maximum unpaired electrons e for a given ligand.
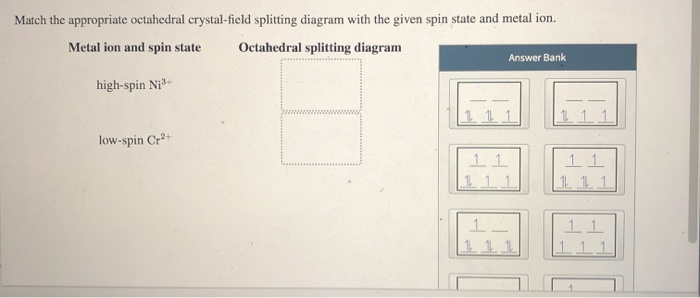
question match the appropriate octahedral crystal field question match the appropriate octahedral crystal field splitting diagram with the given spin state and metal ion high spin cr 2 low spin fe 3 please give the octahedral splitting diagrams for each ion. crystal field stabilization energy Calculation of the LFSE of tetrachloridocobaltate II.
As noted above, eg refers to the ... t2g in octahedral complexes. If the energy required to pair two electrons is greater than Δ, the energy cost of placing an electron in an eg, high spin splitting occurs. The crystal field splitting energy for tetrahedral metal complexes (four ligands) is referred to as Δtet, and is roughly equal to 4/9Δoct (for ...
The difference between the energies of the t 2g and e g orbitals in an octahedral complex is represented by the symbol o.This splitting of the energy of the d orbitals is not trivial; o for the Ti(H 2 O) 6 3+ ion, for example, is 242 kJ/mol. . The magnitude of the splitting of the t 2g and e g orbitals changes from one octahedral complex to another. It depends on the identity of the metal ion ...
Match The Appropriate Octahedral Crystal Field Splitting Diagram Fe4 Crystal field stabilization energies for octahedral complexes four coordinate geometries crystal field theory ffqppor tetrahedra…
Ans. If the element present in high spin …. View the full answer. Transcribed image text: Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. Metal ion and spin state Octahedral splitting diagram Answer Bank high-spin Fe4+ 1 1 1 1 1 1 1 1 low-spin Co2+ 1 1 1 1 1 1 11 1 1 1 1 1 1 1 1 1 1.
It is important to note that the splitting of the d orbitals in a crystal field does not change the total energy of the five d orbitals: the two e g orbitals increase in energy by 0.6Δ o, whereas the three t 2g orbitals decrease in energy by 0.4Δ o. Thus the total change in energy is. (1.2.1) 2 ( 0.6 Δ o) + 3 ( − 0.4 Δ o) = 0.
Answer to Construct the octahedral crystal-field splitting diagram for the metal in each species. V (H2O)63+ Co (CN)63 - Mn (H2O)62+. A d1 octahedral complex is found to absorb visible light, with the absorption maximum occcurring at nm. a) Calculate the crystal-field splitting energy, Δ, in.
Chemistry. Chemistry questions and answers. Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. Metal ion and spin state Octahedral splitting diagram Answer Bank high-spin Ni3+ low-spin Fe4+. Question: Match the appropriate octahedral crystal-field splitting diagram with the given spin state ...
The crystal field stabilization ... ion in the crystal field generated by a set of ligands. It arises due to the fact that when the d orbitals are split in a ligand field, some of them become lower in energy than before. For example, in the case of an octahedron, the t2g set ...
Crystal field splitting diagram for octahedral posted on april 4 2019 by admin image for match the appropriate octahedral crystal field splitting diagram with given spin state the following is a crude and approximate molecular orbital diagram for an octahedral complex octahedral crystal field stabilization energy octahedral splitting with 2. Solved match the appropriate octahedral crystal ...
This photo about: Match the Appropriate Octahedral Crystal-field Splitting Diagram, entitled as Ligand Field Theory Advanced Inorganic Chemistry Lecture Slides Match The Appropriate Octahedral Crystal Field Splitting Diagram - also describes Ligand Field Theory Advanced Inorganic Chemistry Lecture Slides and labeled as: match the following phrases about colour,match the phrases,match the ...
Question match the appropriate octahedral crystal field splitting diagram with the given spin state and metal ion. 3 for each species molecule or ion in the net ionic equation assign oxidation. Solved match the appropriate octahedral crystal field spl match the appropriate octahedral crystal field splitting diagram with the given spin state and metal ion crystal field theory really need ur ...
Sharing MIT's Tradition of Excellence, we commit to changing the world through research, education, and community efforts · 77 Massachusetts Ave., 18-393 | Cambridge, MA 02139 | 617-253-1803 For Emergencies | Accessibility © 2018 MIT Department of Chemistry
CRYSTAL-FIELD SPLITTING DIAGRAMS All four of these transition metals commonly have coordination numbers of \mathbf(6), however, so let's examine their octahedral complex crystal-field splitting diagrams. HIGH SPIN VS. LOW SPIN High spin = fill all five d orbitals with one electron first, and then double up. Low spin = fill lowest-energy d ...
In the model of the crystal field with parameters 10Dq=9500 cm −1 , trigonal splitting C=500 cm −1 , the electronic transition to A g -5 E g at a wavelength of 1.66 μm in the tetrahedron in ...










0 Response to "41 match the appropriate octahedral crystal-field splitting diagram fe4+"
Post a Comment