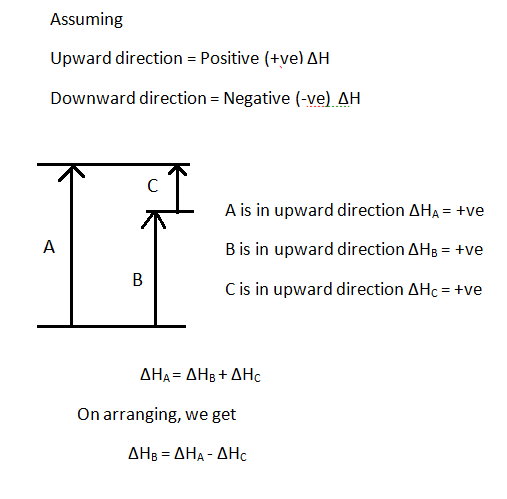
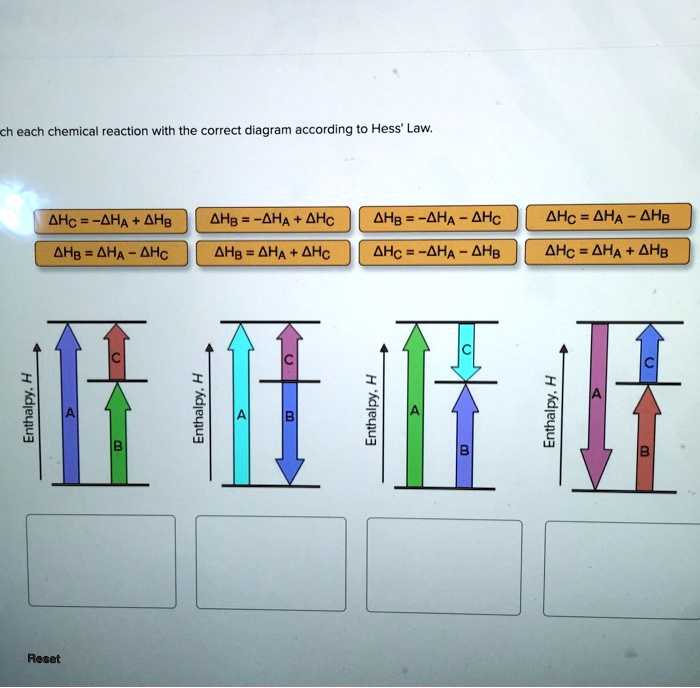
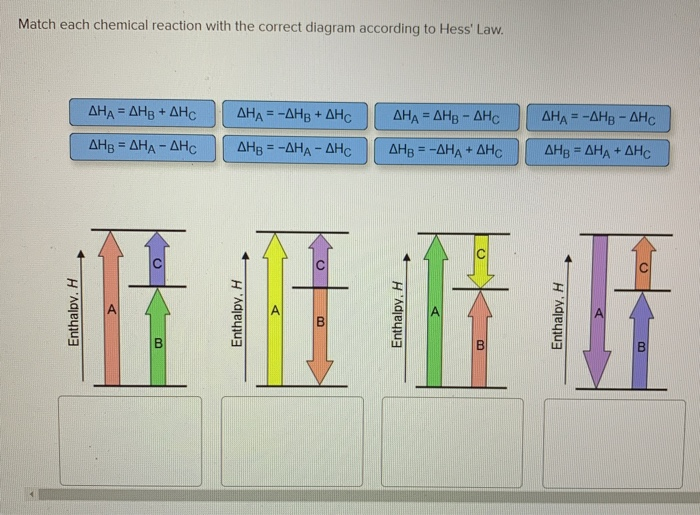
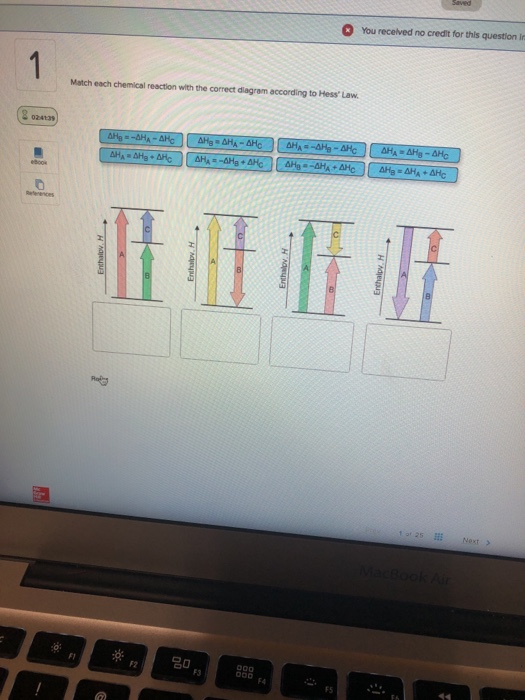
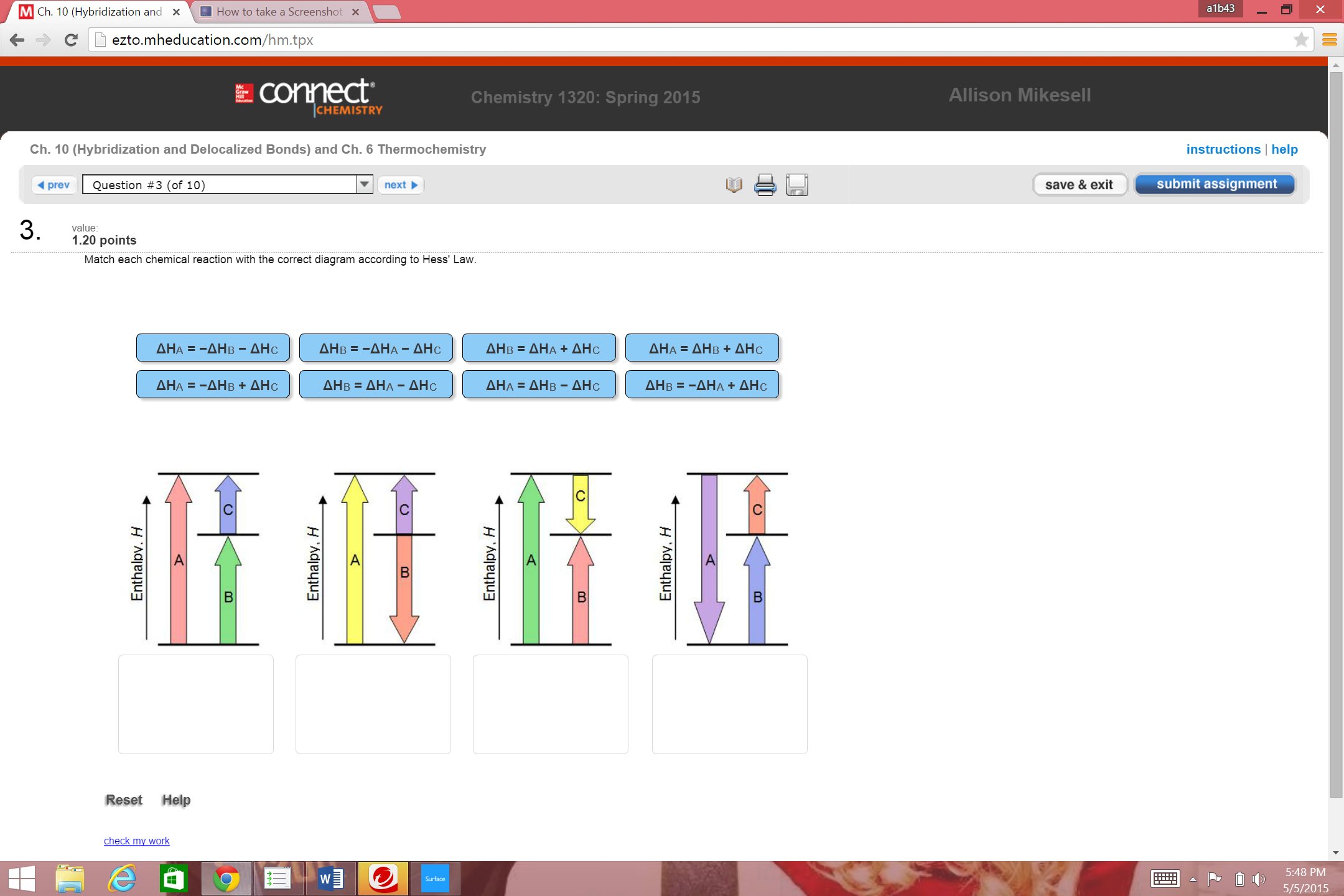
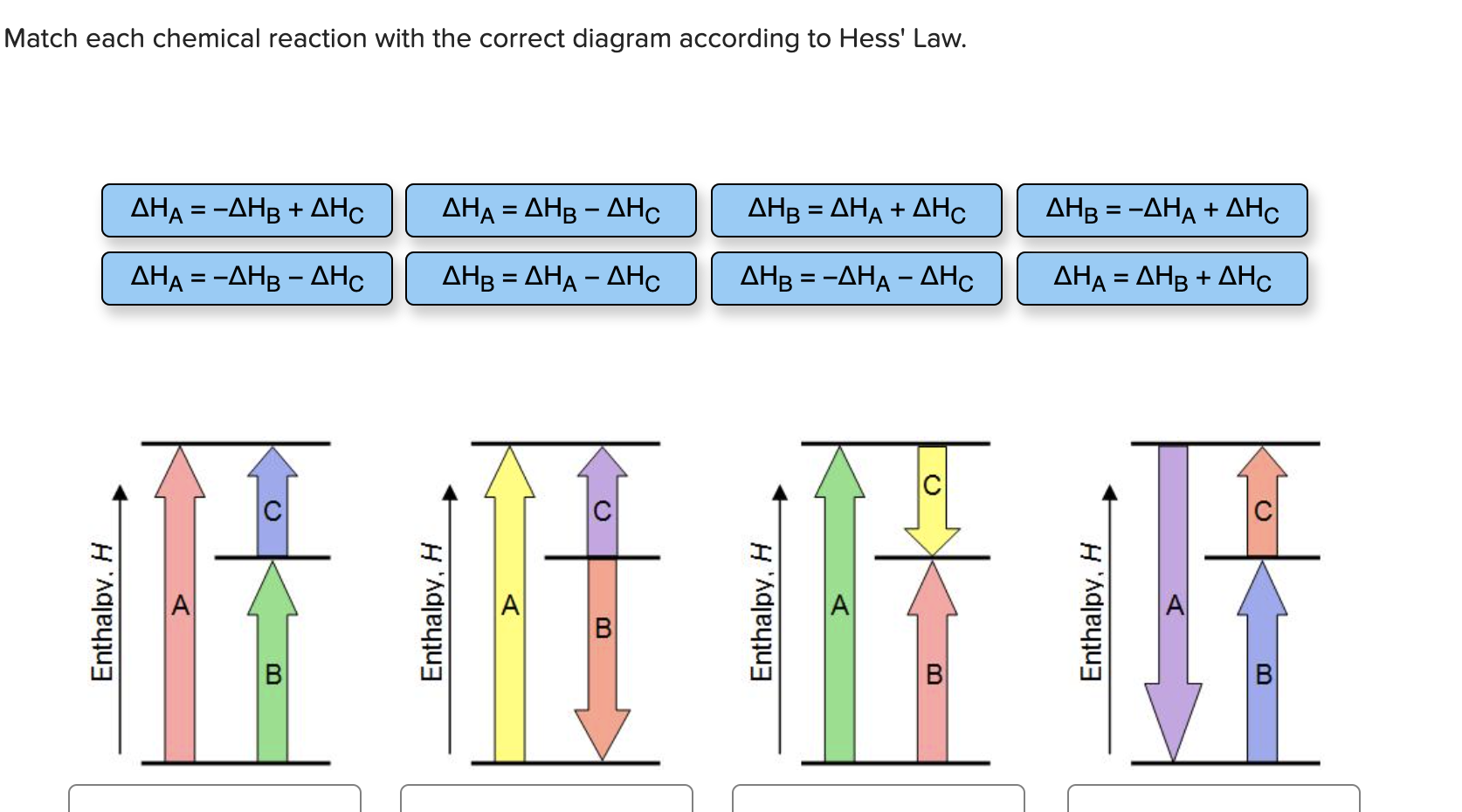
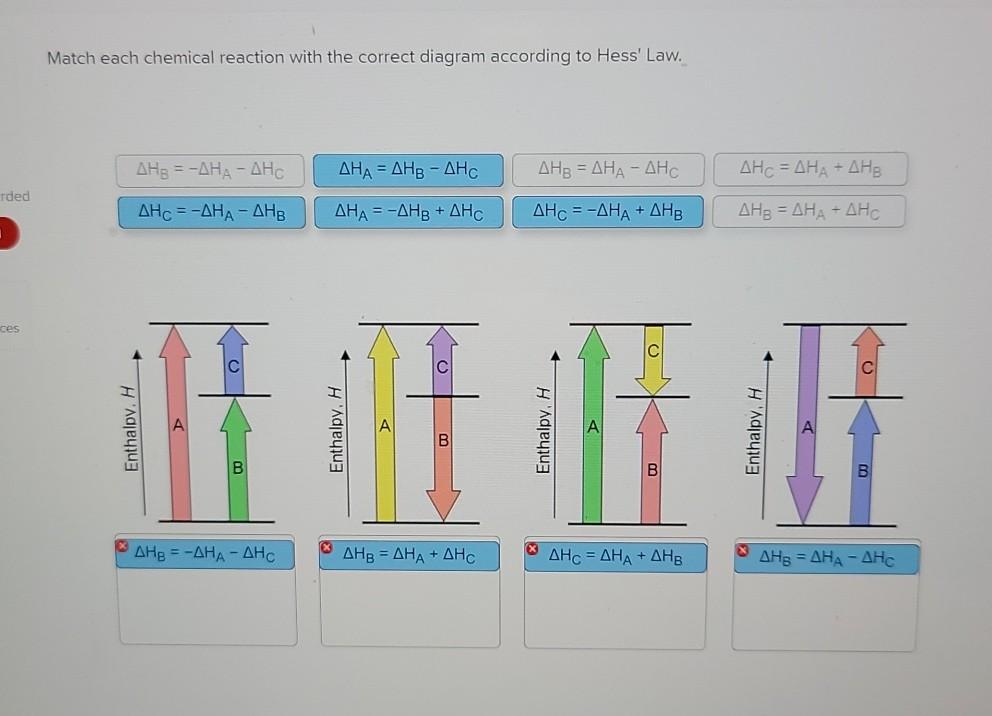
36 match each chemical reaction with the correct diagram according to hess' law.
Hess's law is valid because enthalpy is a state function: Enthalpy changes depend only on where a chemical process starts and ends, but not on the path it takes from start to finish. For example, we can think of the reaction of carbon with oxygen to form carbon dioxide as occurring either directly or by a two-step process.
Transcribed image text: Match each chemical reaction with the correct diagram according to Hess' Law. ΔΗς - ΔΗΚ - ΔΗς ΔΗ. - ΔΗΑ - ΔΗΕ ΔΗ, = -ΔΗΣ - ΔΗ. ΔΗ, = -ΔΗ - ΔΗ- ΔΗ, = - ΔΗΑ ΗΔΗΕ ΔΗς - -ΔΗΣ - ΔΗΕ ΔΗΑ - ΔΗΕ - ΔΗς ΔΗΑ = ΔΗΕ - ΔΗς Enthalpy, H Enthalpy, H B B B ΔΗς = ΔΗΑ + ΔHg ΔΗΑ = ΔΗ + ΔΗ.
The speed of a chemical reaction (a) is constant no matter what the temperature is. ... Given that a reaction absorbs energy and has an activation energy of 50 kJ/mol, which of the following statements are correct? (Hint: Draw the potential energy diagram.) ... According to the mechanism, the rate law will be: (a) Rate = k[A] 2 (b) Rate = k[A][B]
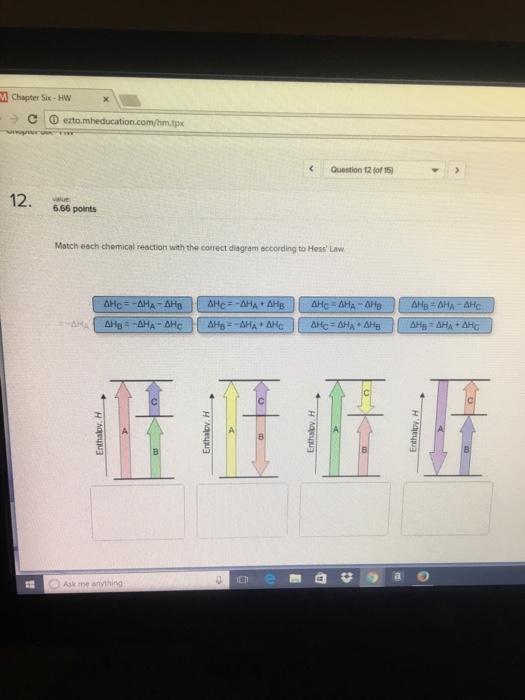
Match each chemical reaction with the correct diagram according to hess' law.
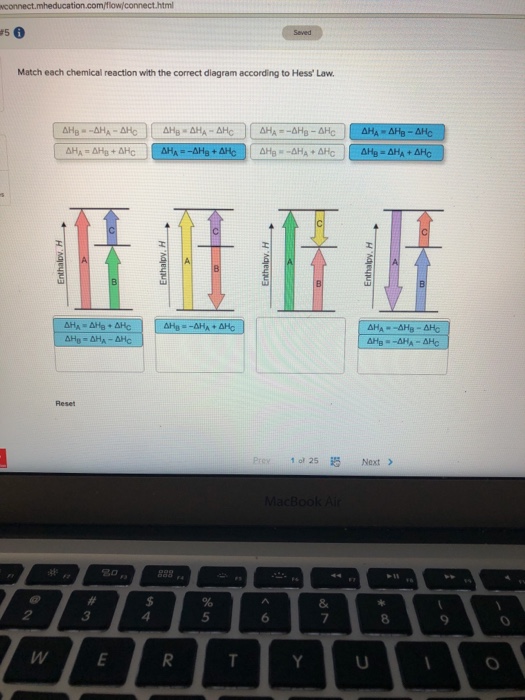
Match each chemical reaction with the correct diagram according to hess law. Here you need to sketch a complete cycle to calculate an enthalpy changes indirectly. The total heat content of the system. Answer to match each chemical reaction with the correct diagram according to hess law.
Answer to match each chemical reaction with the correct diagram according to hess law. That means that if you already know two of the values of enthalpy change for the three separate reactions shown on this diagram the three black arrows you …
As a brief reminder, here is the chemical reaction for the standard enthalpy of glucose: 6C(s, graphite) + 6H 2 (g) + 3O 2 (g) ---> C 6 H 12 O 6 (s) Each standard enthalpy value is associated with a chemical reaction. The reaction will always form one mole of the target substance (glucose in the example) in its standard state.
Match each chemical reaction with the correct diagram according to hess' law..
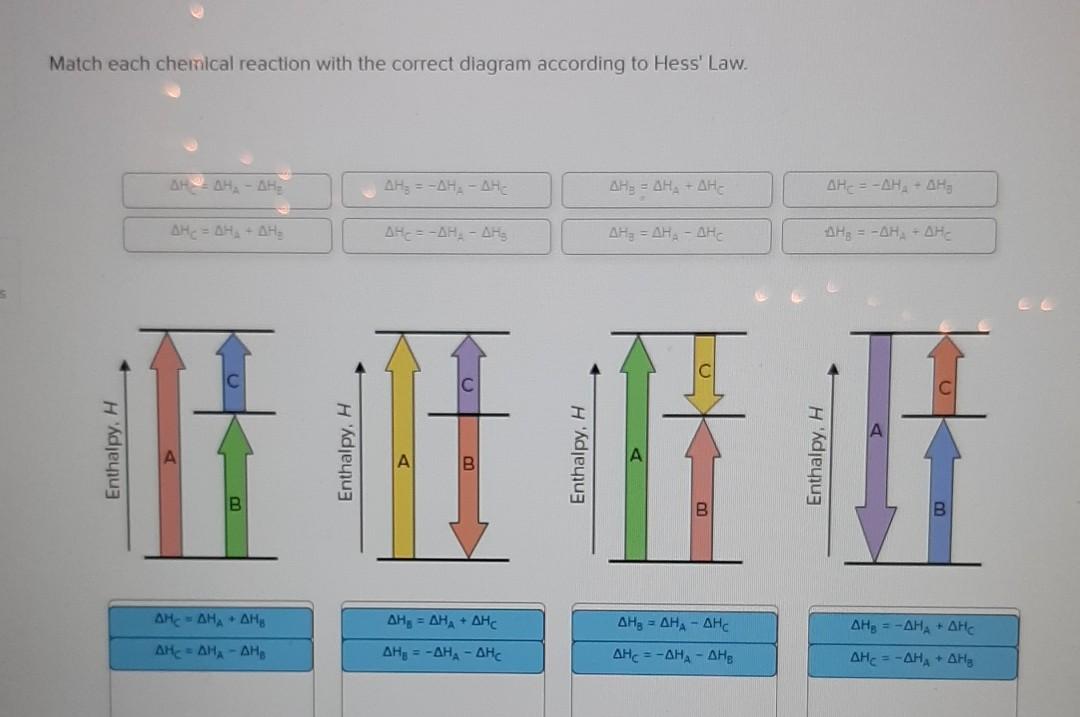
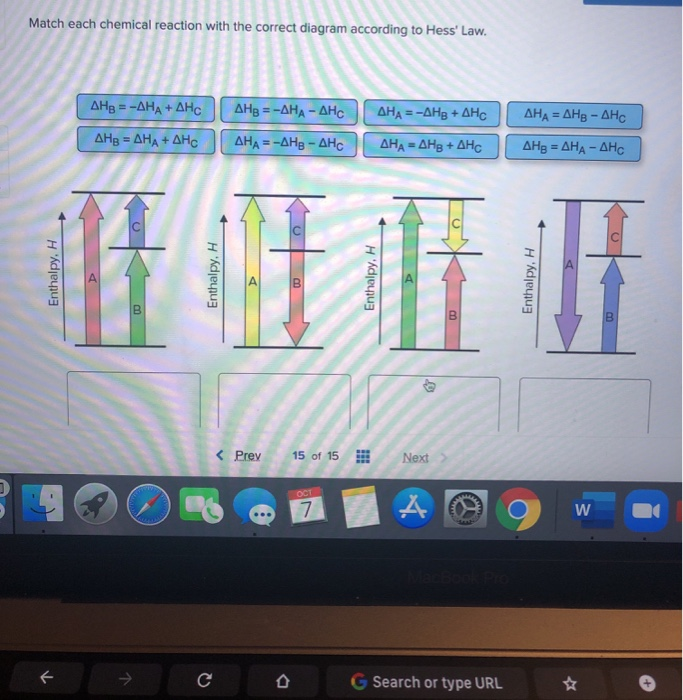
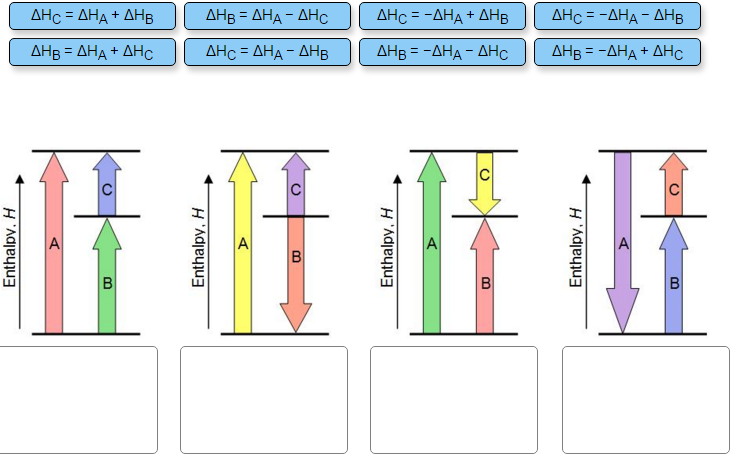
Hess Law of Constant Heat. Answer to 13 Question 13 of 15 points Match each chemical reaction with the correct diagram according to Hess Law. Solving Radical Equations Puzzle Radical Equations Equations Math Tools Match each chemical reaction with the correct diagram according to Hess LawΔHC -ΔHA ΔHB ΔHB ΔHA ΔHCΔHA ΔHB - ΔHCΔHB -ΔHA […]
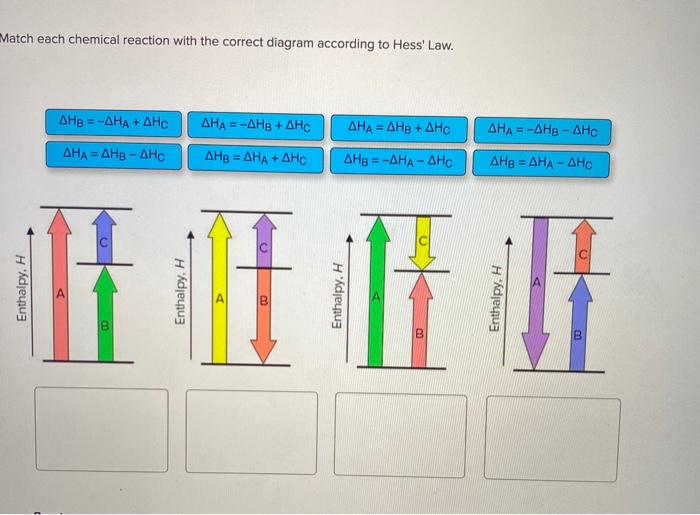
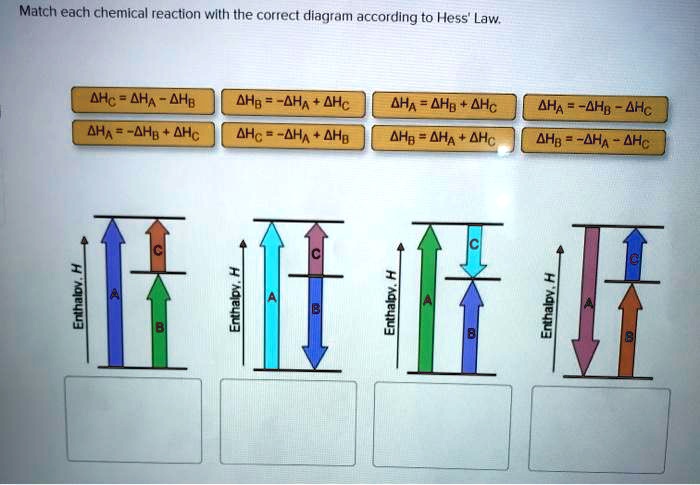
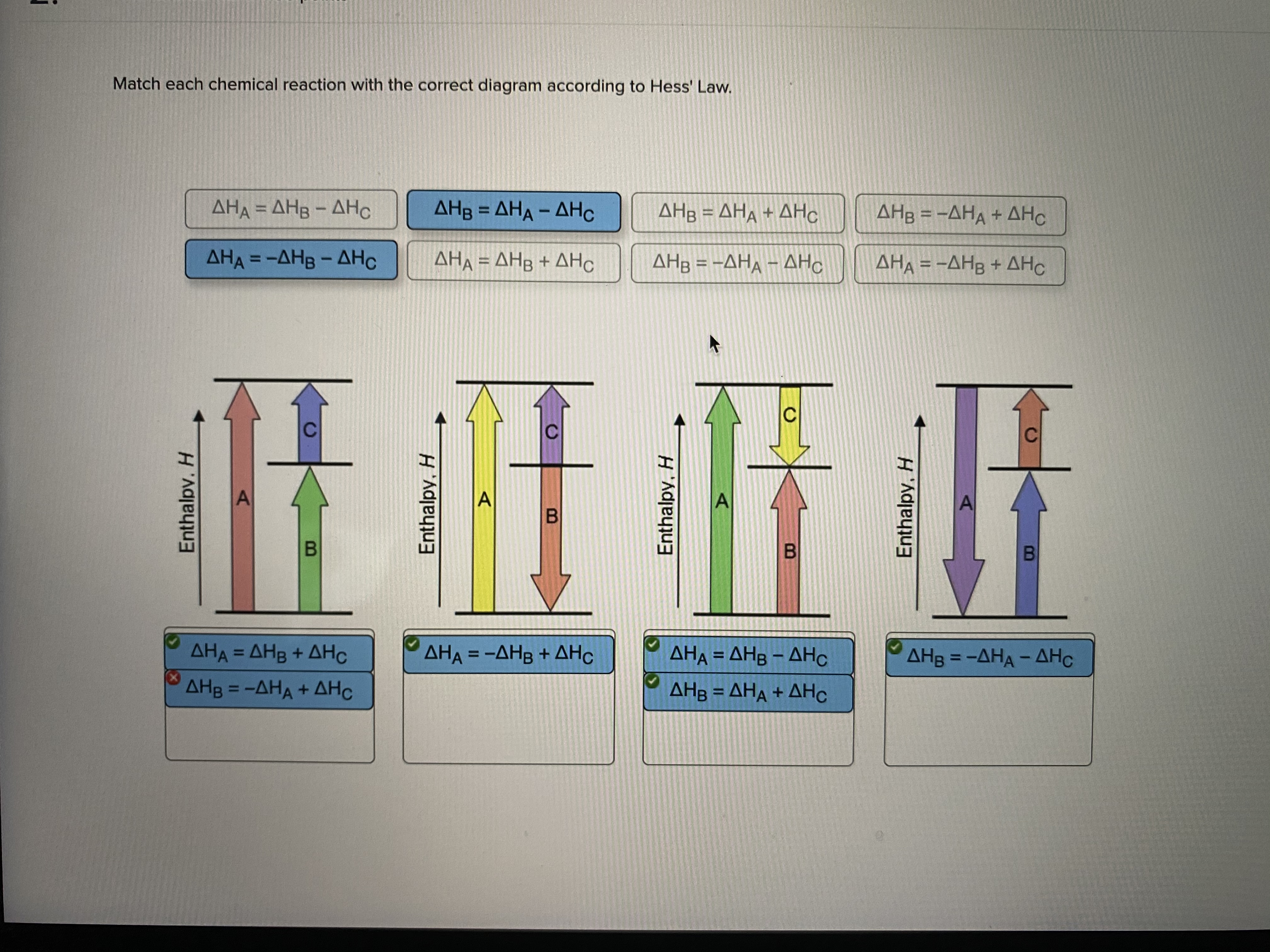
Answered: Match each chemical reaction with the… | bartleby Match each chemical reaction with the correct diagram according to Hess' Law. AHA = AH8 - AHc ΔΗΒ- ΔΗΑ- ΔHC AH3 = AHA + AHc ΔΗΒΔΗΑ AH3 = -AHA + AHC %3D %3D AHA = -AHB - AHC AHA = AH8 + AHo AH3 = -AHA - AHC AHA = -AHg + AHC %3D %3D %3D Question fullscreen Expand Transcribed Image Text
Hess's Law, also known as "Hess's Law of Constant Heat Summation," states that the total enthalpy of a chemical reaction is the sum of the enthalpy changes for the steps of the reaction.Therefore, you can find enthalpy change by breaking a reaction into component steps that have known enthalpy values. This example problem demonstrates strategies for how to use Hess's Law to find the enthalpy ...
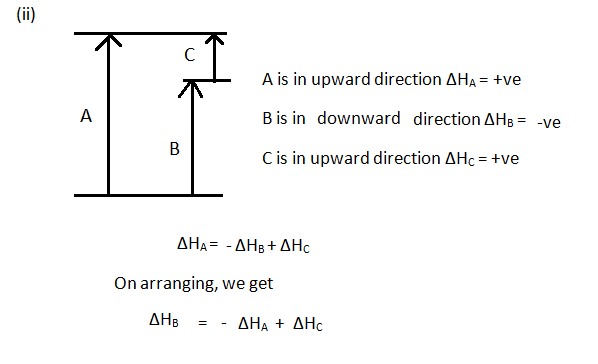
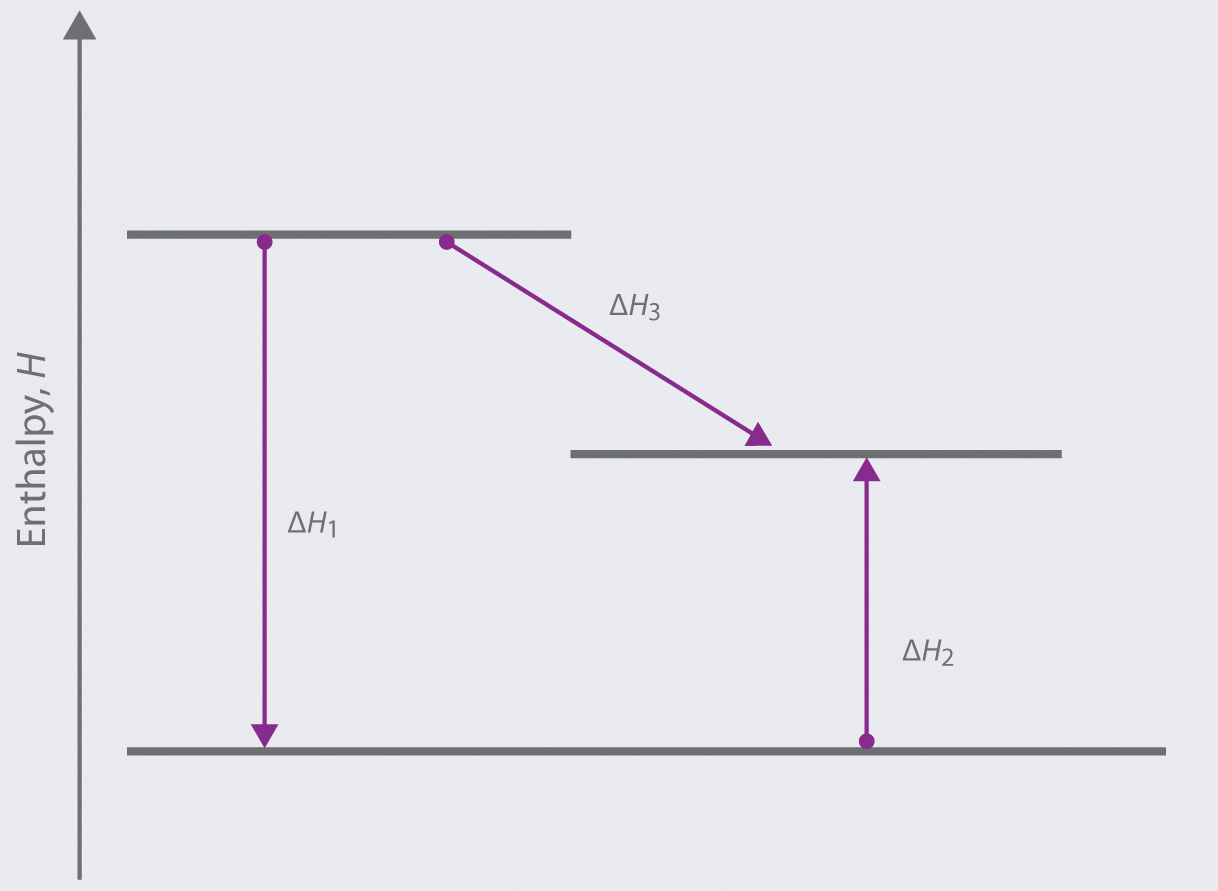
According to Hess's law, if a series of intermediate reactions are combined, the enthalpy change of the overall reaction is the sum of the enthalpy changes of the intermediate reactions. According to the enthalpy diagram below, which of the following statements is true? mc026-1.jpg Arrow C indicates that the third intermediate reaction is ...
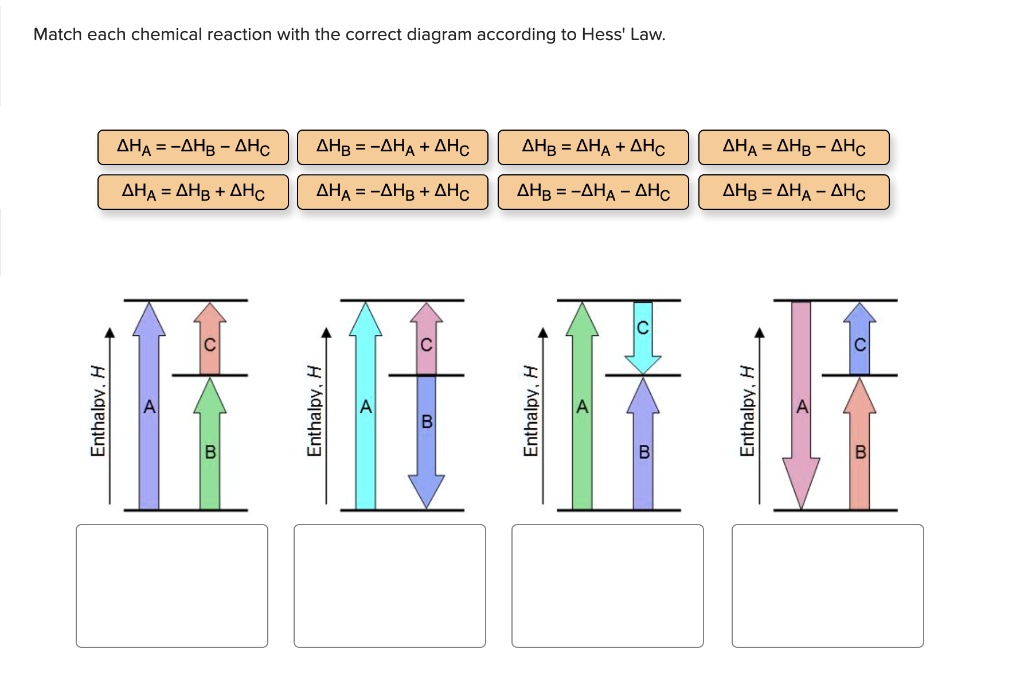
Q. Match each chemical reaction with the correct diagram according to Hess' LawΔHC = -ΔHA + ΔHB ΔHB = ΔHA + ΔHCΔHA = ΔHB - ΔHCΔHB = -ΔHA - ΔHC ΔHA ... Solved • Mar 20, 2020 Hess's Law
13-08-2017 · Match each chemical reaction with the correct diagram according to hess law. The enthalpy of a given chemical reaction is constant regardless of the reaction happening in one step or many steps. Another way to state hess law is. 2h g 2clg h2 cl 2 2hcl g a b δh on an energy level diagram the directions of the.
08-05-2018 · Match each chemical reaction with the correct diagram according to hess law show transcribed image text match each chemical reaction with the correct diagram according to hess law expert answer. Hesss law says that the overall enthalpy change in these two routes will be the same.
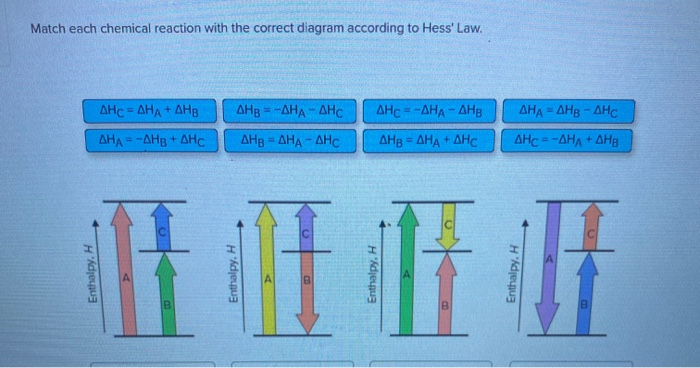
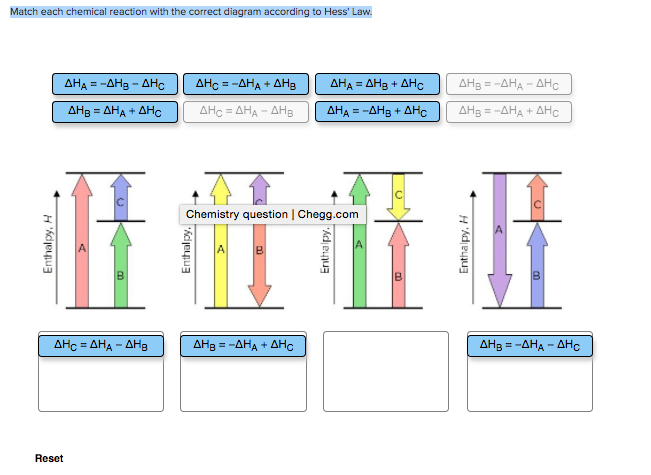
Solved: Match Each Chemical Reaction With The Correct Diag ... Match each chemical reaction with the correct diagram according to Hess' Law. Show transcribed image text. Expert Answer 83% (18 ratings) Previous question Next question Transcribed Image Text from this Question. Match each chemical reaction with the correct diagram according to ...
Another way to state Hess' Law is The enthalpy change of an overall process is the sum of the enthalpy changes of its individual steps. The process needed to answer the question above is based on the principle that if you add two or more equations to get a new equation, you must add the ΔH's to get the ΔH for the new equation.
Problem Details. Match each chemical reaction with the correct diagram according to Hess' Law. ΔH C = -ΔH A + ΔH B. ΔH B = ΔH A + ΔH C. ΔH A = ΔH B - ΔH C. ΔH B = -ΔH A - ΔH C. ΔH A = -ΔH B + ΔH C. ΔH C = ΔH A + ΔH B. ΔH B = ΔH A - ΔH C.
mc019-2.jpg. What is the correct enthalpy diagram using the Hess law for this system? A. Jason combines the two intermediate steps shown. mc027-1.jpg. Which best describes what Jason should do with the oxygen molecules? D. If a chemical reaction is reversed, what happens to the enthalpy of the reaction? C.
Hess's Law is the most important law in this part of chemistry. Most calculations follow from it. It says . . . The enthalpy change accompanying a chemical change is independent of the route by which the chemical change occurs. Hess's Law is saying that if you convert reactants A into products B ...
Hesss law also known as hesss law of constant heat summation states that the total enthalpy of a chemical reaction is the sum of the enthalpy changes for the steps of the reaction. Match each chemical reaction with the correct diagram according to hess law. Learn vocabulary terms and more with flashcards games and other study tools.
ENE‑3.D (LO) Transcript. Hess's law can be used to calculate enthalpy changes that are difficult to measure directly. In this video, we'll use Hess's law to calculate the enthalpy change for the formation of methane, CH₄, from solid carbon and hydrogen gas, a reaction that occurs too slowly to be measured in the laboratory. Created by Sal Khan.
these reactions: C(s) + O2(g) → CO2(g) ΔHo = -393.5 kJ CO(g) + ½ O2(g) → CO2(g) ΔHo = -283.0 kJ According to Hess's Law, we can combine the above two reactions in a manner that will give the desired reaction. Note that if we reverse the second reaction and add it to the first reaction, we will obtain the
Hess' Law describes the conservation of energy. That is, that regardless of the path taken during a chemical reaction or whether the chemical reaction was completed in one step or several, the enthalpy change in the reaction remains the same.
Enthalpy and Thermochemical Reactions. Enthalpy and Thermochemical Reactions. Physical and chemical changes are done under constant pressure. Gained or lost heat in reactions under constant pressure is called enthalpy change.Enthalpy is the total kinetic and potential energy of particles of matter.
Hess's law of constant heat summation, also known as Hess' law, is a relationship in physical chemistry named after Germain Hess, a Swiss-born Russian chemist and physician who published it in 1840. The law states that the total enthalpy change during the complete course of a chemical reaction is independent of the number of steps taken.. Hess' law is now understood as an expression of the ...
The enthalpy of a given chemical reaction is constant, regardless of the reaction happening in one step or many steps. Another way to state Hess' Law is: If a chemical equation can be written as the sum of several other chemical equations, the enthalpy change of the first chemical equation equals the sum of the enthalpy changes of the other ...
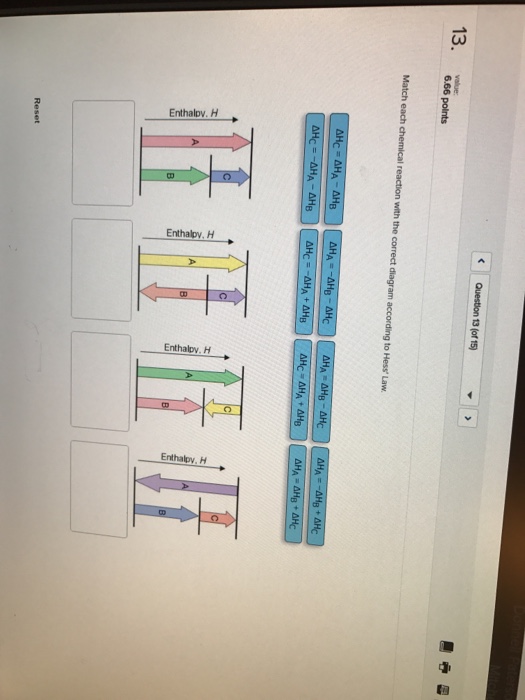
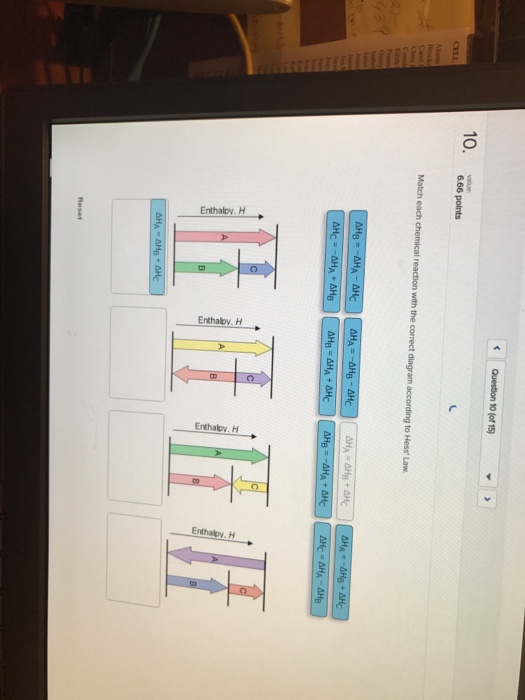
22-01-2019 · Answer to 13 Question 13 (of 15) points Match each chemical reaction with the correct diagram according to Hess Law. A Reset. Hess' Law of Constant Heat Summation The enthalpy of a given chemical reaction is constant, regardless of the reaction happening in one step or many Another way to state Hess' Law is: 1) Analyze what must happen to each equation.
Different reaction pathways can be represented on the same energy diagram to represent the application of Hess's Law. Hess's Law can be used to calculate the overall enthalpy change for a chemical reaction, the enthalpy changes for each individual reaction during the process are added together (that is, summed). Steps involved in calculating ...
Match each description below to one of the reactions sets (A, B, C or D) from Model 1. ... What general type of reaction is illustrated in the diagram? Write the correct formula for the product of this reaction. ... Write a formula equation for each chemical reaction. 1 1) Calcium reacts with hydrochloric acid. 12) Pentane, C5H12, burns in the ...
Match each chemical reaction with the correct diagram according to hess' law. Energy can, however, be transferred from one substance (or system) to ano the r, or it can change form. Examples of energy transformations include a light bulb (electricity to light and heat), an exo the rmic chemical reaction like combustion ( chemical potential to ...
Law of Conservation of Energy. ... Calculate the heat in Kj associated with 361 grams of white phosphorus burning in air according to the equation P4(s) + 5O2(g) →P4O10(s) ... Match each chemical reaction with the correct diagram according to Hess' Law... On Exam #3 draw on flash card. (#7)
Step by Step: Hess's Law (see at end for supplemental notes on ∆H formation with Hess's Law) The enthalpy change (ΔH r o) for a reaction is the sum of the enthalpy changes for a series of reactions, that add up to the overall reaction. Steps: For each reaction: 1) Check to see, if the compounds are on the correct sides of the reaction.
12. Hess's law of heat summation is not related to the law of conservation of enerw. 13. When using Hes" law of heat summation. interrnediate reactions are summed and terms are canceled. as in algebra, to arrive at a final equation. 14. _TI 5. The for and H20(s) are the same. Part C Matching Match each description in Column B the correct term ...
Ch each chemica reaction with the correct diagram according to hess law ahc aha ahb ahb aha ahc ahb aha ahc ahc aha ahb ahb aha ahc ahb aha ahc ahc aha ahb ahc aha ahb j reaet 25805
60 MHR Chemistry 12 Solutions Manual 978 --07-106042-4 Using Hess's Law to Determine Enthalpy Change (Student textbook page 316) 41. Nitrogen dioxide, NO 2(g), is an emission resulting from the burning of gasoline in an automobile engine that contributes to the formation of smog and acid rain.
See the answer. See the answer See the answer done loading. Match each chemical reaction with the correct diagram according to Hess' Law. Show transcribed image text.
09-11-2019 · Match each chemical reaction with the correct diagram according to hess law. You may be given combustion data or formation data. Delta ha delta hb delta hc delta h. Terms in this set 10 consider the following intermediate reactions. 1 check to see if the compounds are on the correct sides of the reaction.
So if we want the reaction from A. To see if we simply add these up, then the bees will cancel out like an algebra and then has his losses that we can also add the heats delta H one plus delta H. Two which is 60 minus 90 or 30 negative killer jewels for the reaction. And that completes friday for part B. Let's superimpose the entropy diagrams and show how has this law applies here.
27-11-2020 · Match each chemical reaction with the correct diagram according to Hess' Law. points Print References Reset



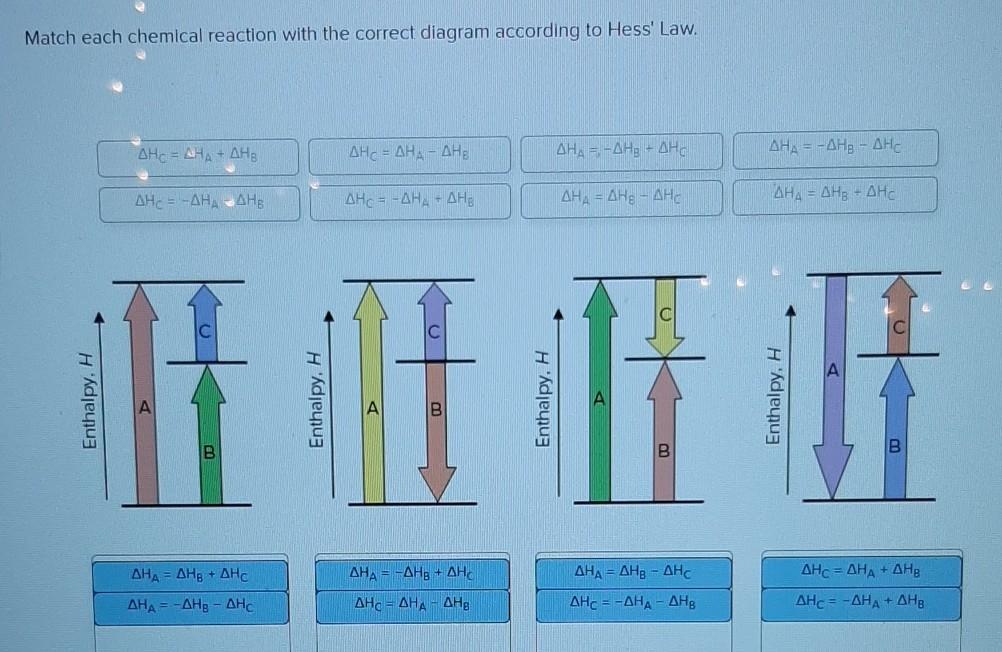






0 Response to "36 match each chemical reaction with the correct diagram according to hess' law."
Post a Comment