40 fluorine electron dot diagram
What is the Lewis dot structure for fluorine? To figure out the Lewis dot structure, look at the valence electrons. These are electrons in the outermost shell. 1. Figure out the group it is in at ... A Lewis structure shows two fluorine atoms, each with three lone pairs of electrons,. 5. Rearrange the electrons of the outer atoms to make multiple bonds with ...
Beryllium fluoride (BeF2) lewis dot structure, molecular geometry, electron geometry, polar or nonpolar, bond angle. Beryllium fluoride is an inorganic compound that appears as colorless lumps have a chemical formula BeF2. It is an odorless white solid also known as fluoride salt of beryllium. It is commonly used in biochemistry.
Fluorine electron dot diagram
schematron.org The resulting lewis electron dot structure is: Lesen Sie auch: 10+ 5 Relay Stabilizer Circuit. Source: d2vlcm61l7u1fs.cloudfront.net. Fluorine is the most electronegative element, and so therefore, for silicon tetrafluoride, we're going to put the silicon atom at the center of our dot structure, since it is the least electronegative of those two. (d) The Lewis electron-dot diagram for C2H4 is shown below in the box on the left. In the box on the right, complete the Lewis electron-dot diagram for C2H50H by drawing in all of the electron pairs. H ë:: H H ë H 1 point is earned for a correct diagram. Diagram should include all bonding pairs plus two nonbonding pairs on the O atom.
Fluorine electron dot diagram. Nov 23, 2021 · Steps to form OF2 Lewis Structure Diagram. Step 1: Find the Total number of Valence Electrons. The first and foremost step is to calculate the total number of valence electrons in an OF2 molecule. Oxygen belongs to group 16, the chalcogen family, and has a valency of 6. Fluorine belongs to the family of halogen in group 17 and has a valency of 7. F2O or OF2 lewis dot structure, molecular shape, electron geometry, polar or non-polar, bond angle Home > Chemistry Article > OF2 lewis structure and its molecular geometry/shape Oxygen difluoride is a colorless and very toxic gas with the chemical formula OF2. 7 dots would be placed around fluorine in a Lewis electron dot diagram. In Lewis structure, we represent valence electrons by dots and bonds by line.. Atomic number of fluorine is 9. Electronic configuration is 2,7. So 7 valence electrons are present in fluorine. Drawing the Lewis Structure for F 2. Viewing Notes: F 2 is a reddish gas at room temperature. The F 2 Lewis structure is similar to Br 2, Cl 2, and I 2 since F, Br, Cl, and I are all in Group 7 and have 7 valence electrons. For the F 2 Lewis structure there are a total of 14 valence electrons available.
(d) Xenon can react with oxygen and fluorine to form compounds such as XeO 3 and XeF 4. In the boxes provided, draw the complete Lewis electron-dot diagram for each of the molecules represented below. XeO 3 XeF 4 One point is earned for each correct Lewis electron-dot diagram. Omission of lone pairs of electrons on the O or F atoms A step-by-step explanation of how to draw the F2 Lewis Dot Structure (Diatomic Fluorine).Note that Diatomic Fluorine is often called Molecular Fluorine or ju... Fluorine is in Group 17 of the Periodic Table..... And thus the neutral atom has 7 valence electrons. Of course the elemental form is bimolecular. Upon reduction, the fluorine atom forms fluoride, which has 8 valence electrons, and is isoelectronic with a Noble Gas (which one?). Which do you think would be bigger; fluorine atom or fluoride ion? Fluorine's Diagram. ... Mass Number: 18.9984032 Electron configuration: 1s2,2s2,2p5 or [HE] 2s2 2p5 Valence Electrons: 7 Orbital Notation: Electron Dot Notation: Powered by Create your own unique website with customizable templates. Get Started ...
Fluorine is the most electronegative element, and so therefore, for silicon tetrafluoride, we're going to put the silicon atom at the center of our dot structure, since it is the least electronegative of those two. Pictorial Electron dot structure - valence electrons are represented by dots placed around the. Draw a Lewis electron dot diagram for an atom or a monatomic ion. In almost all Fluorine and neon have seven and eight dots, respectively: Fluoride-Neon. A larger outer circle has one red dot on, representing the second shell with one electron. Lewis Dot Diagram Worksheet Use the Bohr models to determine the number of valance electrons. Once you have found the number of valance electrons, place them around the elements symbol. Element Atomic # Atomic Mass Protons Neutrons Electrons Lewis Dot Carbon 6 12 6 6 6 l Hydrogen 1 1 1 0 1 H Lithium 3 7 3 4 3 Li Fluorine is the ninth element with a total of 9 electrons. In writing the electron configuration for fluorine the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for F go in the 2s orbital. The remaining five electrons will go in the 2p orbital. Therefore the F electron configuration will ...
Fluorine Dot Diagram. lewis dot structure for fluorine atom f a step by step explanation of how to draw the lewis dot structure for f fluorine i show you where fluorine is on the periodic table and how what is the lewis electron dot diagram for a socratic fluorine is in group 17 of the periodic table and thus the neutral atom has 7 valence electrons course the elemental form is bimolecular
What is the Lewis dot structure of fluorine? The diatomic fluorine molecule (F2) contains a single shared pair of electrons. Each F atom also has three pairs of electrons that are not shared with the other atom. A lone pair is a pair of electrons in a Lewis electron-dot structure that is not shared between atoms.
Solution. Having lost its two original valence electrons, the Lewis electron dot diagram is just Ca 2+. Ca2+. The O 2− ion has gained two electrons in its valence shell, so its Lewis electron dot diagram is as follows: Test Yourself. The valence electron configuration of thallium, whose symbol is Tl, is 6 s2 5 d10 6 p1.
Draw a Lewis electron dot diagram for an atom or a monatomic ion. In almost all Fluorine and neon have seven and eight dots, respectively: Fluoride-Neon.The Lewis Structure (electron dot diagram) of each ion is used to construct the Lewis Structure (electron dot diagram) for the ionic compound. Examples. Lithium fluoride, LiF.
2. The Lewis Structure (electron dot diagram) of each ion is used to construct the Lewis Structure (electron dot diagram) for the ionic compound. Lithium fluoride, LiF 1. Lithium atom loses one electron to form the cation Li + 2. Fluorine atom gains one electron to form the anion F-3. Lithium fluoride compound can be represented as Li + OR 1.
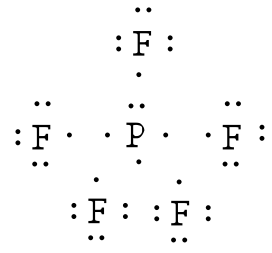
Lewis dot structure for PF3. As you see in this PF3 lewis dot structure, phosphorous and fluorine completed their octet, and everything looks fine, but, for the sake of satisfaction, we should also determine the formal charge in the above structure to know whether it is stable or not. 6. Check the stability with the help of a formal charge concept
Fluorine requires only one valence electron in order to complete its octet. Therefore, two atoms of fluorine form covalent bond with each other. In the representation of the electron dot structure of ${{\rm{F}}_{\rm{2}}}$, the valence electrons are represented by dots around each fluorine atom.
How did Rutherford figure out the structure of the atom without being able to see it? Simulate the famous experiment in which he disproved the Plum Pudding model of the atom by observing alpha particles bouncing off atoms and determining that they must have a small core.
Electron dot diagram of Fluorine atom. Electron dot diagram also called lewis structure which represents the valence electrons of atoms. As, from the Bohr diagram of Fluorine, we got to know, it has 7 valence electrons. So, just represent these 7 valence electrons around the Fluorine atom as a dot.
Lewis Structure (electron dot diagram) for hydrogen fluoride OR The 2 electrons making up the bonding pair of electrons between the hydrogen atom and the fluorine atom, which may or may not be circled, are referred to as a covalent bond (or a single covalent bond).Electron Dot StructuresWhat is the Lewis dot structure for fluorine.
31 Jan 2013 — The diatomic fluorine molecule (F2) contains a single shared pair of electrons. Each F atom also has three pairs of electrons that are not ...
Lewis Structure (electron dot diagram) for hydrogen fluoride OR The 2 electrons making up the bonding pair of electrons between the hydrogen atom and the fluorine atom, which may or may not be circled, are referred to as a covalent bond (or a single covalent bond).
A step-by-step explanation of how to draw the Lewis dot structure for F (Fluorine). I show you where Fluorine is on the periodic table and how to determine ...
Nov 21, 2021 · In the outermost shell, we have 6 electrons that are also known as valence electrons in the case of Fluorine. Lewis Dot Structure of SF6. The central atom here is Sulfur as it is less electronegative than Fluorine. This is because the outer shell of Fluorine has 5 electrons and it needs one more electron to reach stability, which is easier to ...
Review what a Lewis dot diagram is and discover how to draw a Lewis dot structural formula for compounds. Learn how to represent single, double and triple bonds with lines instead of dots. Also ...
Fluorine electron configuration is 1s 2 2s 2 2p 5.The symbol for fluorine is F. The period of fluorine is 2 and it is a p-block element. The electron configuration of fluorine(F) and the orbital diagram is the main topic of this article.
Using Lewis dot diagrams, show how some number of atoms of magnesium and atoms of fluorine can transfer electrons to form ions of each element with stable.What is the correct lewis electron-dot structure for the compound magnesium fluoride? Chemistry Covalent Bonds Drawing Lewis Structures.
Match each element to its electron dot diagram. The symbol X represents the element. Refer to the periodic table if needed. fluorine sodium phosphorus magnesium One of the X's didn't work, but it had 2 dots on top, 2 on the right, 2 on the bottom, and 1 on the left.
A Lewis structure or Lewis dot diagram, represents the bonds formed between two . Lewis Dot notation is a way of describing the outer shell (also called the valence shell) of an atom's electrons. Dots are Examples are Fluorine and Sulfur. Lewis The ions are arranged in a crystalline structure with each Na+ ion attracted to.
What is the correct Lewis dot structure for fluorine? The question says,'which is the correct Lewis structure for fluorine, which is a group 7A element. Lewis structure is used to represent the covalent bonding of molecules. Flourine has seven electron in its outermost shell, so the Lewis structure of flourine will have 3 lone pair of dots ...
(d) The Lewis electron-dot diagram for C2H4 is shown below in the box on the left. In the box on the right, complete the Lewis electron-dot diagram for C2H50H by drawing in all of the electron pairs. H ë:: H H ë H 1 point is earned for a correct diagram. Diagram should include all bonding pairs plus two nonbonding pairs on the O atom.
The resulting lewis electron dot structure is: Lesen Sie auch: 10+ 5 Relay Stabilizer Circuit. Source: d2vlcm61l7u1fs.cloudfront.net. Fluorine is the most electronegative element, and so therefore, for silicon tetrafluoride, we're going to put the silicon atom at the center of our dot structure, since it is the least electronegative of those two.
schematron.org



0 Response to "40 fluorine electron dot diagram"
Post a Comment