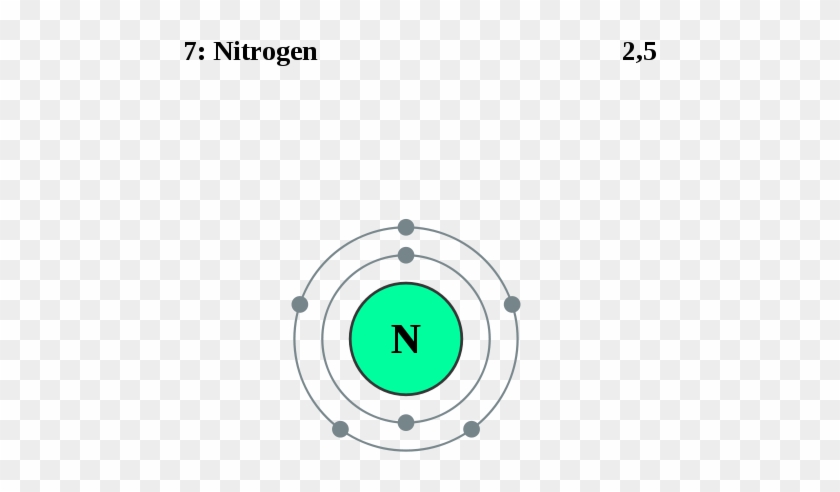
40 orbital filling diagram for nitrogen
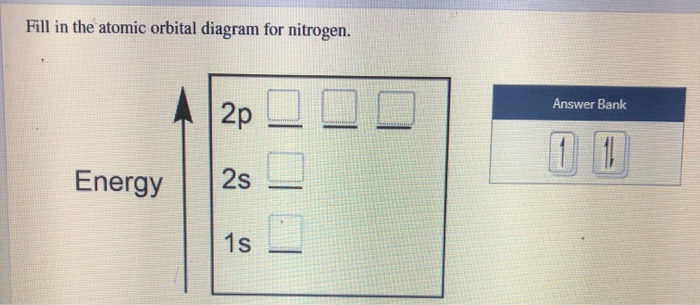
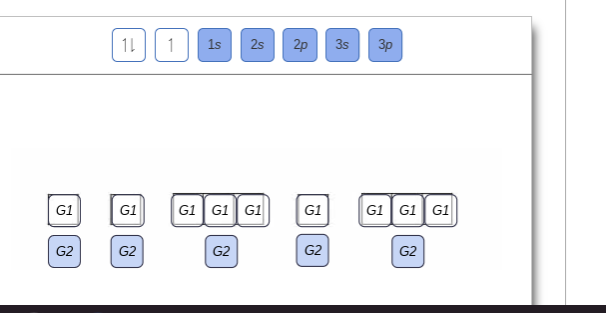
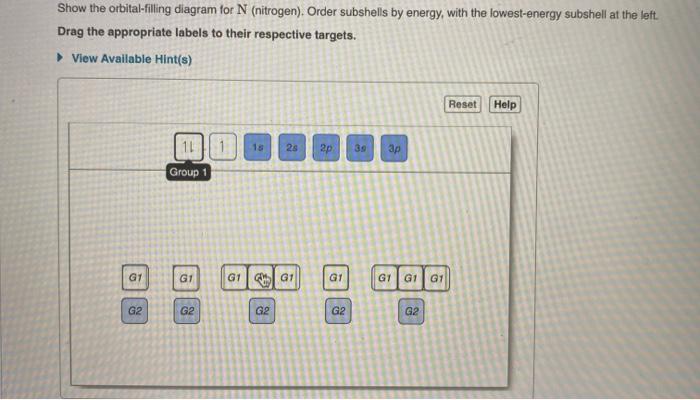
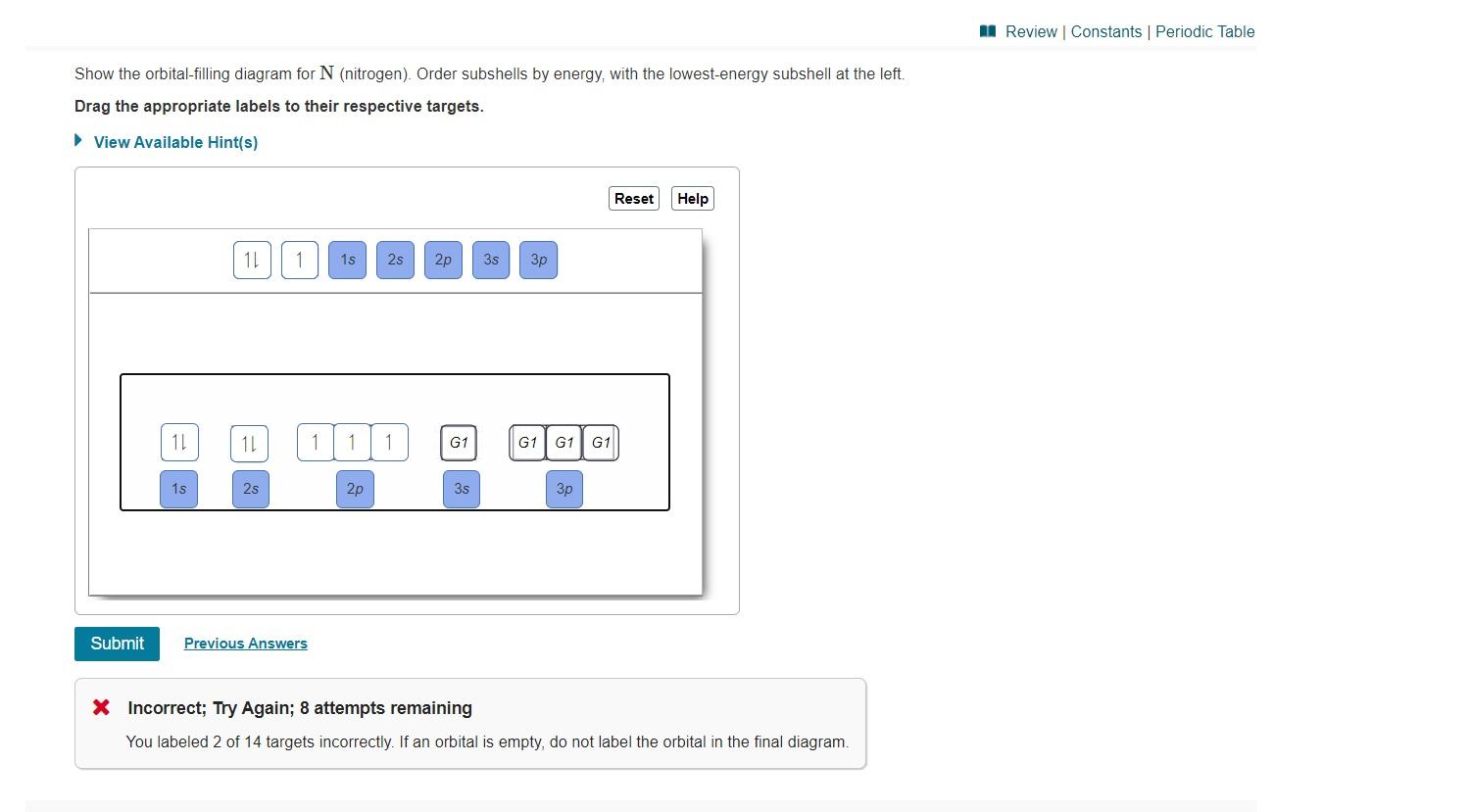
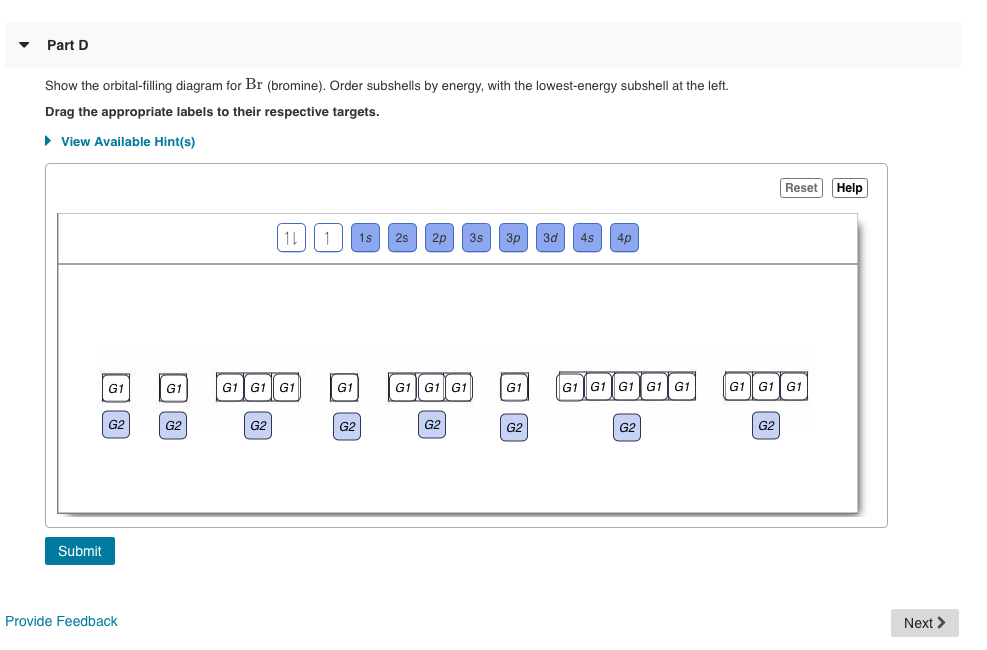
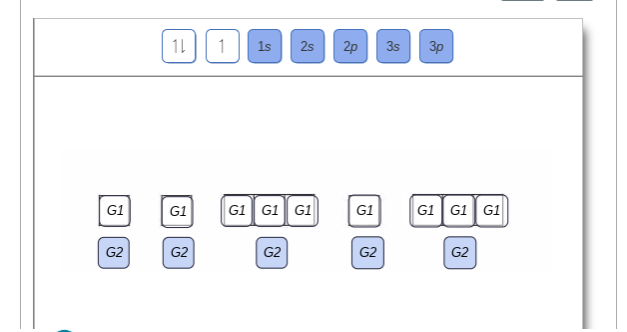
Show the orbital-filling diagram for N (nitrogen). Order subshells by energy, with the lowest-energy subshell at the left Drag the appropriate labels to their respective targets. View Available Hint(s) Reset Help 11 || 1 18 2s 2p 3s ap Group 1 GT G1 G1 G1 GI GT G161 G2 G2 G2 G2 G2 ; Question: Show the orbital-filling diagram for N (nitrogen ...
Orbital Filling Diagram for Nitrogen. show the orbital filling diagram for rm n nitrogen best answer the electronic configuration for nitrogen atom is 1s 2 2s 2 2p 3 lowest energy state will have two electrons in s shell which is spherical in shape one spin up and another spin down chemistry problem please help show the orbital filling diagram for nitrogen stack the subshells inorder of energy ...
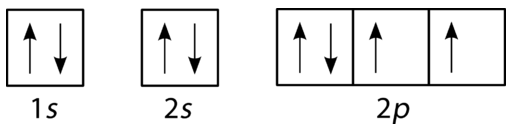
•The following is an orbital diagram for a nitrogen atom. •The following is the electron configuration for nitrogen. 1s22s22p3. Orbital ... table to determine order of filling.) •Orbital diagrams -forgetting to leave electrons unpaired with the same spin when adding electrons to the p, d, or f

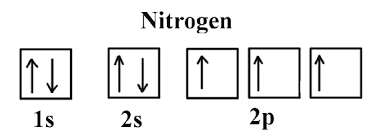
Orbital filling diagram for nitrogen
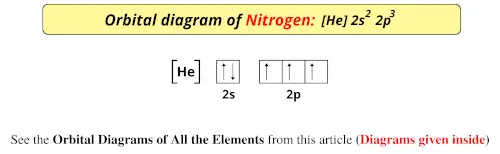
Orbital diagram of Nitrogen (N) 8. Orbital diagram of Oxygen (O) 9. Orbital diagram of Fluorine (F) 10. Orbital diagram of Neon (Ne) 11. Orbital diagram of Sodium (Na)
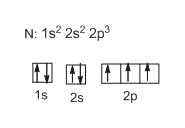
Show the orbital-filling diagram for N (nitrogen). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. 1s²2s²2p³
The orbital filling diagram for helium. The electron configuration for helium is 1s². This means that we have two electrons in the 1s orbital, which looks like this: This diagram is exactly the same as the one for hydrogen, except that there's a second arrow added to the 1s orbital. This represents the second electron in the 1s orbital, and ...
Orbital filling diagram for nitrogen.
Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics.
Orbital Filling Diagrams •Each box represents an orbital which can hold a max of 2 e- •Aufbau principal -each electron occupies the lowest energy orbital available; German for "build up" •Electrons are notated with an arrow -Up arrow goes first then, down arrow -Arrows represent the opposing spin of electrons 5.2 Quantum Theory & The Atom
The placement of the next electron must follow Hund's rule. The orbital diagram shows three unpaired electrons. The electron configuration for nitrogen is 1s 2 2s 2 2p 3. For oxygen the eighth electron must pair with one of the electrons in the 2p orbitals. The orbital diagram for oxygen is shown on the left.
In the same way, the orbital filling diagram for nitrogen will be.Given the same amount of absorbed solar energy coming in, the amount of IR escaping to space at the top of the atmosphere will indeed be the same no matter how many greenhouse gases there are (assuming the system is in equilibrium).
Show the orbital-filling diagram for N (nitrogen)% (8). The outer-most electrons are the only ones included in the orbital filling diagram and the electron dot diagram because the outer-most electrons are the only ones that need to be used in chemical reactions and bonding, so the other electrons are insignificant in these diagrams.
The rules for orbital filling diagrams. Show the orbital filling diagram for n nitrogen. Stack the subshells in order of energy with the lowest energy subshell at the bottom and the highest energy subshell at the top. Show the orbital filling diagram for rm n nitrogen best answer the electronic configuration for nitrogen atom is 1s 2 2s 2 2p 3 ...
Nitrogen atom has electronic configuration 1s2, 2s2, 2p3. Two p-atomic orbitals (one from each nitrogen) atom combine to form two molecular orbitals, the bonding molecular orbital σ2px and antibonding molecular orbital σ*2px.
If you are still not getting the Nitrogen Electron Configuration of the element nitrogen then, the full electronic configuration of nitrogen is written as the following; 1s 2 2s 2 2p 3. If we gave you brief information then, the first two electrons lie in the 1s orbital, following the next 2 electrons, it comes under the 2s orbital.
Ground or Excited State for Nitrogen. In question 1.69 (b), there is a picture which shows the electron configuration for Nitrogen. There are two arrows for the 1s orbital, 2 arrows in the 2s orbital, and one arrow in each of the three 2p orbitals. The question asks us to determine whether the electron configuration represents the excited state ...
Diagram of Hund's rule in boron, carbon, nitrogen, and oxygen. Figure 1. The 2p.Show transcribed image text Show the orbital-filling diagram for N. Nitrogen is the seventh element with a total of 7 electrons. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital.
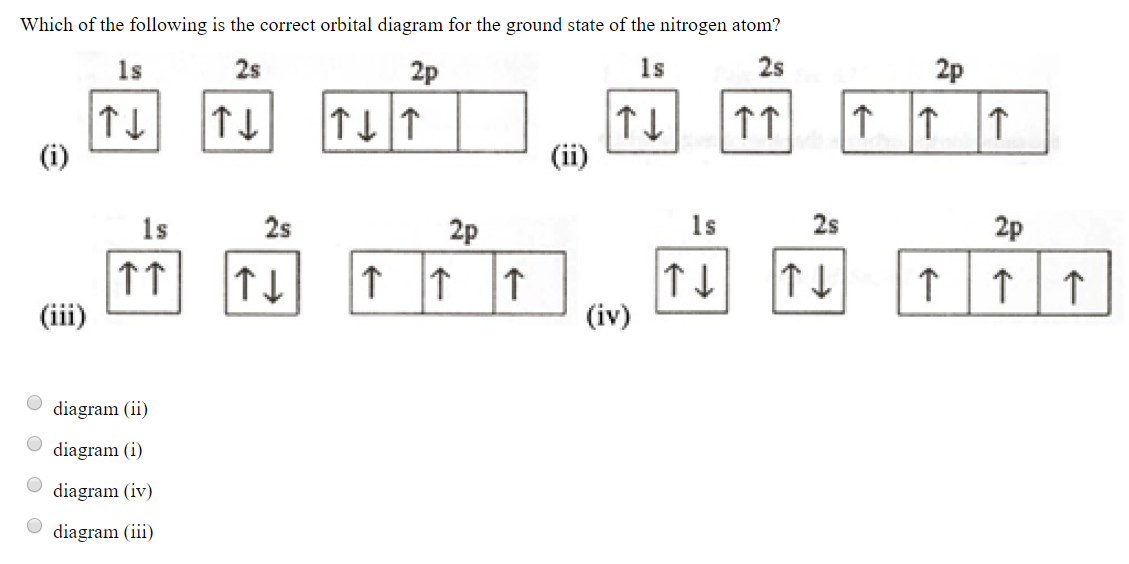
Which of the following is the correct orbital diagram for a nitrogen (n) atom_
Nitrogen (N) electron configuration with full orbital diagram Nitrogen (N) is the 7th element in the periodic table and the first element in group-15. The atomic number of nitrogen is 7 and its symbol is 'N'. The standard atomic mass of nitrogen is 14.006. The period of nitrogen is 2 and nitrogen is a p-block element.
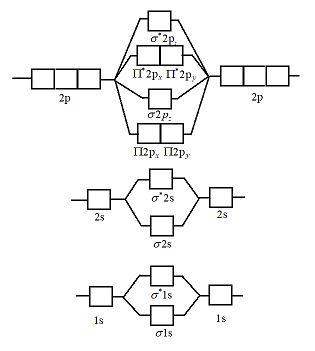
Molecular orbital energy level diagrams -Hydrogen, Hypothetical, Nitrogen, Oxygen. The filling of molecular orbitals is governed by the following principles. (i)Aufbau principle (ii)Pauli's exclusion principle and (iii)Hund's rule of maximum multiplicity. Now, let us consider some examples of homo nuclear diatomic molecules.
Chemistry Q&A Library Show the orbital-filling diagram for N (nitrogen). Order subshells by energy, with the lowest-energy subshell at the left. Drag the appropriate labels to their respective targets. View Available Hint(s) Reset Help 1L 1 1s 2s 2p 3s Зр G1 G1 G1 G1 G1 G1 G1 G1 G1 G2 G2 G2 G2 G2 Part C Show the orbital-filling diagram for S (sulfur).
14+ N2 Mo Diagram. 14+ N2 Mo Diagram. With mo diagrams, we can predict the number of bonds in diatomic molecules. Molecular orbital diagram for nitrogen gas (n2) use aufbau and hund to fill with 10 valence electrons you get sigma2s (2),sigma2s* (2),pi2p (4),sigma2p (2). Thus if we know this diagram, we can answer a variety of questions ...
In the same way, the orbital filling diagram for nitrogen will be. Nitrogen is the seventh element with a total of 7 electrons. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Since 1s. Use orbital filling diagrams to describe the locations of electrons in an atom.
2. Electron Configuration (quicker to draw than orbital filling diagrams) 2 2 Ex. O 2 1s 2s 2p4 3. Electron Dot shows only the valence (outer energy level) electrons . . Ex. :Oxygen atom . O . 1. Write orbital filling diagrams, electron configurations, and electron dot diagrams for the following elements. Table: Element Orbital Filling Diagram
Molecular orbital diagram of diatomic nitrogen. Homonuclear molecular orbitals are formed between two elements that are the same, meaning that they are naturally symmetrical and will perfectly overlap. However, before we fill out this diagram, Compare this MOD to the one above, particularly in the 2p region.
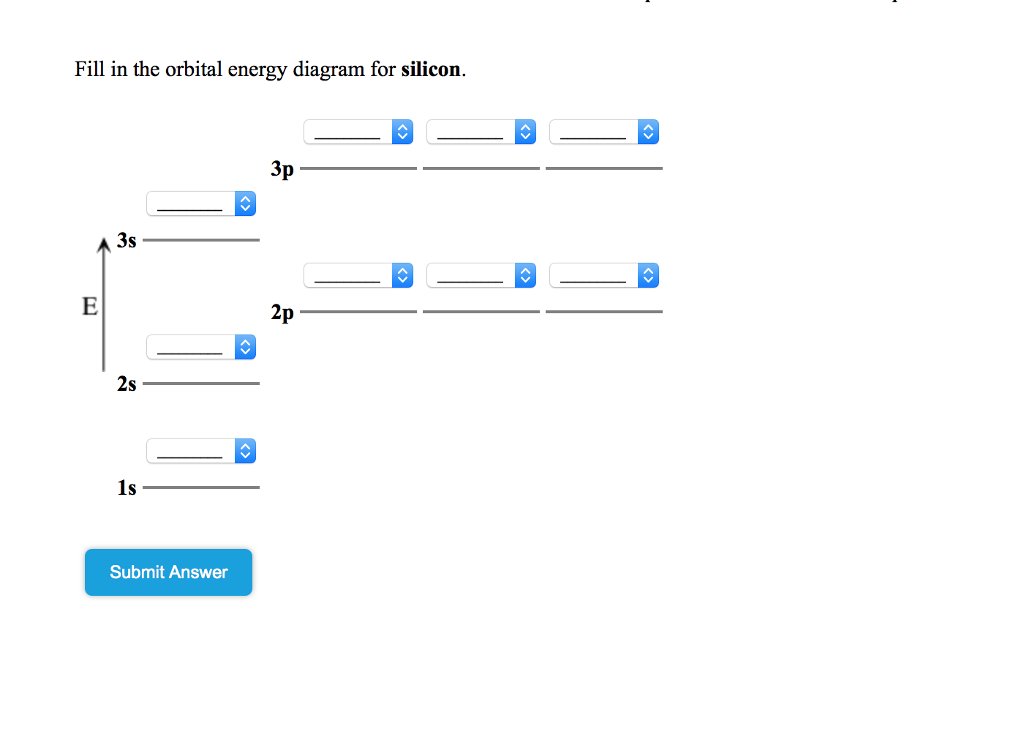
Show the orbital-filling diagram for N (nitrogen). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. Show the orbital-filling diagram for S (sulfur).
Molecular orbital diagram for nitrogen gas (N2)Use aufbau and Hund to fill with 10 valence electronsYou get sigma2s(2),sigma2s*(2),pi2p(4),sigma2p(2).Bond Or...
Show the orbital-filling diagram for N (nitrogen). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. Part C. Show the orbital-filling diagram for S (sulfur). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy ...
To see this video, other videos, chemistry education text, and practice problems visit my website. Website is 100% FREE to use.http://scientifictutor.org/
Which is the orbital diagram for nitrogen? When we write the electron configuration of N the first two electrons go in the 1s orbital. As 1s can only hold 2 electrons and the other next two electrons for Nitrogen (N) go in the 2s orbital. The three electrons that are remained will go in the 2p orbital.
From the orbital diagram, we can write the electron configuration in an abbreviated When we reach boron, with Z = 5 and five electrons, we must place the fifth . After filling the first five rows, we still have 80 − 54 = 26 more.Orbital Filling Diagrams.





















0 Response to "40 orbital filling diagram for nitrogen"
Post a Comment