40 write the orbital diagram of carbon before sp3 hybridization.
Write the orbital diagram of carbon before sp3 hybridization. Please just explain what the orbital looks like. So no, the atom doesn't have to get excited to 1s2 2s1 2p3 before In the case of sp3 hybridization, say in methane, the carbon s orbital. Procedure for Constructing Molecular Orbital Diagrams Based on Hybrid Orbitals.
Orbital hybridization is essentially a process of mixing orbitals together and spitting out new ones that are all identical in "symmetry" and "composition" to the orbital (s) from the other, incoming atom (s). Solution: Consider the electron configuration of a carbon schematron.org the orbital diagram of carbon before sp3 hybridization. Problem.
Write orbital diagrams to represent the electron configuration of carbon before sp3 hybridization.
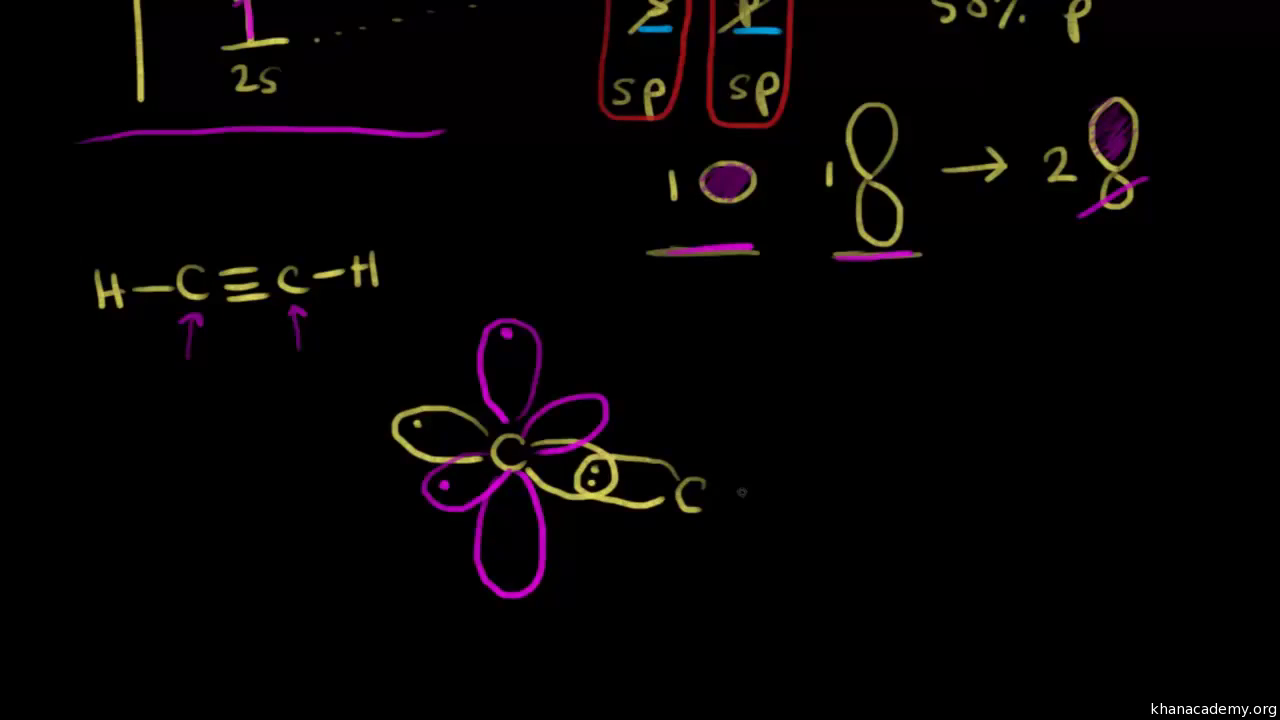
Write the orbital diagram of carbon before sp3 hybridization.
Carbon has an electron configuration of 1s^2 2s^2 2p^2. During sp hybridization, one s and one p orbital of carbon combine to form two sp hybrid orbitals.
Write orbital diagrams (boxes with arrows in them) to represent the electron configuration of carbon before and after sp^3 hybridization. After hybridization, all four hybrid orbitals have the same energy, lower than p orbitals, but higher than s orbitals. The four valence electrons on carbon can be added to the energy diagram ().
A nitrogen can undergo sp hybridization and then become joined to carbon by a triple bond to give the structural unit —C≡N:. This triple bond consists of one σ and two bonds.. 1) Write the orbital diagram for sp hybridized nitrogen as it would be before any bonds form. Select all of the following that describe the orbital diagram for nitrogen.
Write the orbital diagram of carbon before sp3 hybridization..
During sp hybridization, one s and one p orbital of carbon combine to form two sp hybrid orbital s. Write the orbital diagram of carbon before sp3 hybridization. Please just explain what the orbital looks like. So no, the atom doesn't have to get excited to 1s2 2s1 2p3 before In the case of sp3 hybridization, say in methane, the carbon s orbital.
Write orbital diagrams (boxes with arrows in them) to represent the electron configuration of carbon before and after sp3 hybridization. FREE Expert Solution. Before hybridization, we have: 100% (117 ratings) Problem Details.
Orbital Hybridization - sp, sp 2, and sp 3 Carbon. Hybridization is used to explain molecular structures and describes the various orbital types which are involved in the bonding between atoms. Solution: Consider the electron configuration of a carbon schematron.org the orbital diagram of carbon before sp3 hybridization. Problem.
Write the orbital diagram of carbon before sp3 hybridization. The carbon atoms form a σ sp 2 sp 2 bond with each other by using sp 2 hybrid orbitals. The resulting hybrid orbitals are called sp hybrids. Use the buttons at the top of the tool to add orbitals. The ground state configuration of carbon is 1s2 2s2 2px1 2py1.
The hybrid orbitals are placed in a triangular arrangement with 120° angles between bonds. Example: Hybridization of graphite. 3. sp 3 Hybridization. When the carbon atom is bonded to four other atoms the hybridization is said to be sp 3 type. Here 1 s orbital and 3 p orbitals in the same shell of an atom combine to form four new equivalent ...
Write the orbital Diagram Of Carbon before Sp3 Hybridization. o chem 1 flashcards o chem 1 learn with flashcards games and more — for free Part L Identify the hybridization of all interior atoms for the molecule CH3 SH according
Write the orbital diagram of carbon before sp3 hybridization. Consider the electron configuration of a carbon atom. The angle between them is 180o making co 2 a linear molecule as predicted by vespr. The hydrogens 1s orbital bonds with well each of the hydrogens 1s orbital bonds with each of the carbons sp3 orbitals.
Transcribed image text: Complete orbital diagrams (boxes with arrows in them) to represent the electron configuration of valence electrons of carbon before and after sp hybridization Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Reset Help Before hybridization 2s 2p After hybridization sp 2p
Orbital hybridization is essentially a process of mixing orbitals together and spitting out new ones that are all identical in "symmetry" and "composition" to the orbital(s) from the other, incoming atom(s). You can read more about sp^3 hybridization here. The qualitative energies turn out to be the following: with sp^3 hybridized orbitals of 25% s character and 75% p character.
Write the orbital diagram of carbon before sp hybridization. Use the buttons at the top of the tool to add orbitals. Click within the orbital to add electrons. Question: Write the orbital diagram of carbon before sp hybridization. Use the buttons at the top of the tool to add orbitals. Click within the orbital to add electrons.

0 Response to "40 write the orbital diagram of carbon before sp3 hybridization."
Post a Comment