37 the partial (valence-level) orbital diagram for a 3+ ion is shown below.
What is the sum of the numbers of neutrons and electrons in the ion 28P1- ? 29. ... In the energy diagram shown below, the electron is shown (as a red dot) starting in the n = 3 level . ... Give the ground-state electron configuration, symbol, and group number from each partial (valence-level) orbital diagram. The partial (valence-level) orbital diagram for a 2+ ion is shown below. Write the symbol of the element from which this ion is derived: Write the formula of the oxide this ion forms: Question: The partial (valence-level) orbital diagram for a 2+ ion is shown below. Write the symbol of the element from which this ion is derived: Write the ...
a. the n = 3 shell has no f subshell b. there are three p orbitals in every shell of an atom except the n = 1 shell c. all s orbitals have spherical shapes d. each d subshell has five d orbitals e. the energies of subshell in the shells (energy levels) of a hydrogen atom vary as s < p < d, etc.
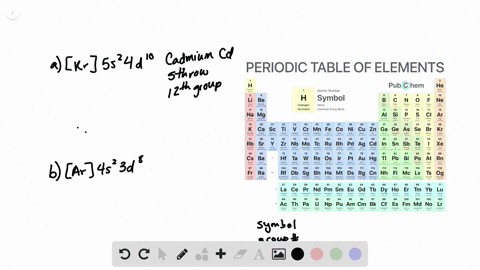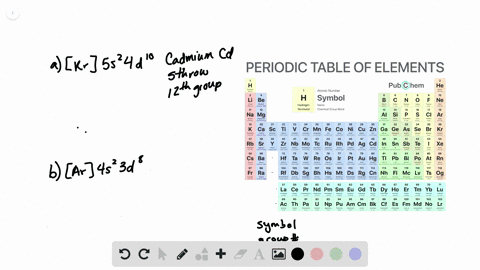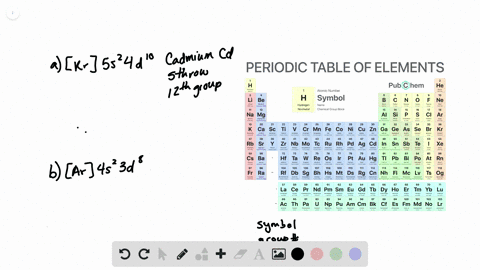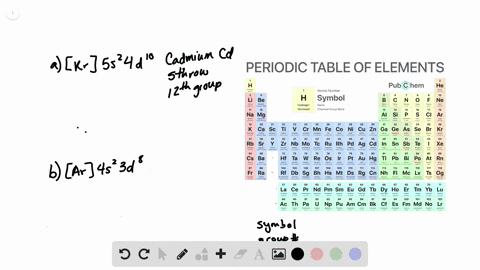
The partial (valence-level) orbital diagram for a 3+ ion is shown below.
The partial (valence−level) orbital diagram for a 2+ ion is shown below. What is the symbol of the element from which this ion is derived? A. Ti B. Zr C. Cr D. Mo E. Ru Answer: B. This 2+ ion has two electrons in the 4d set. Since the charge of the ion is 2+, two electrons were removed from the atom to make the ion. Transcribed image text: The partial (valence-level) orbital diagram for a 3+ ion is shown below. Write the symbol of the element from which this ion is ... 5.13 The energy level diagram for SH- is shown below. A bond order of 1 is predicted. The S orbital energies are -22.7 eV (3s) and -11.6 eV (3p); the 1s of H has an energy of -13.6 eV. Because of the difference in their atomic orbital energies, the 1s orbital of hydrogen and the
The partial (valence-level) orbital diagram for a 3+ ion is shown below.. M.O.Energy Level Diagram for A2 (A = Li, Be) Li2 Only two valence electrons, i.e. σs 2σ*s 0. Bond order = 1. Diamagnetic Li2 exists in gas phase over metallic lithium. "Be2" σ s 2σ* s 2 B o ndr e= 0 - t b i g energy, so molecule does not exist. Beryllium in gas phase is monatomic. Use Aufbau, Pauli, Hund - just as in filling atomic orbitals Best Answer: look at the number of valence electron here. it's on the orbital number 5, having a total of 4 valence electrons (outermost shell electrons) that will contribute to bonding. So with F having a -1 charge. And E will have to be a cation of +4. So total is EF4. a) The 1s orbital in H is more stable than the 1s orbital in He+ b) The 2s orbital in He atom is less stable than the 2s orbital in He+ c) The 2s subshell in Li is more stable than the 2p subshell in Li a. a) only b. a) and b) only c. a) and c) only d. c) only e. None of these The partial (valence-level) orbital diagram for a 3-ion is shown below. What is the symbol of the element from which this ion is derived? 个 4s 3d ND Cr TI OV Mn Co ; Question: The partial (valence-level) orbital diagram for a 3-ion is shown below. What is the symbol of the element from which this ion is derived? 个 4s 3d ND Cr TI OV Mn Co
The partial (valence-level) orbital diagram for a 3+ ion is shown below.__ 4s (no arrows) __ __ __ _ 3. How many types of isotopes do you have? 4. When potassium chlorate is heated, it decomposes into potassium, chlorine and oxygen. How many gram; 5. 1. Calculate the potential for the titration of 40.0 mL of 0.10 M Fe2+ with 5.0, 10.0, 20.0 and ... From the partial (valence-level) orbital diagram, write the ground-state electron configuration and ; 3. Calculate the approximate volume of a 0.62 mol sample of gas at 10.6 °C and a pressure of 1.05 atm. 4. The partial (valence-level) orbital diagram for a 3+ ion is shown below.__ 4s (no arrows) __ __ __ _ 5. How many types of isotopes do you ... Calculate the approximate volume of a 0.62 mol sample of gas at 10.6 °C and a pressure of 1.05 atm. Shouldn’t you count the valence electrons for Be (which is 2) and subtract 1 because of the + sign? For O2, N2, NO, F2, etc, you count the number of valence electrons instead of the atomic number. Why is it that for Be, though, you look at the atomic number instead of the number of valence electrons it has? I apologize if this is a stupid question, but I appreciate any clarification on this
Ions also have electron configurations (Section 7.4). Cations have fewer valence electrons, and anions have more valence electrons, respectively, than their parent atoms. For example, chloride, Cl-, has an electron configuration of 1s22s22p63s23p6, for a total of 18 electrons, compared to 17 for neutral chlorine, the element. Na has an ... MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine So the formal it'll be cobalt thio, oxygen three. So here and see were given an empty five s orbital and a completely filled in 40 orbital with 10 electrons in it. And we're told that the iron has a charge of plus one. This one's a little tricky because usually want the s orbital be completely filled before you fill in the d orbital. Consider the following portion of the energy-level diagram for hydrogen: n = 4-0.1361 × 10-18 J n = 3-0.2420 × 10-18 J n = 2-0.5445 × 10-18 J n = 1-2.178 × 10-18 J For which of the following transitions does the light emitted have the longest wavelength?
Jan 27, 2022 · In the hydrogen atom the three sublevels, 3s, 3p and 3d were all degenerate in energy. Click here👆to get an answer to your question ️ The following question relates to the partial energy level diagram for the hydrogen electron: .54eV n5 .85eV n4 1.51eV n3 3.4eV n2 13.6eV n1 The question relates to electron located at E3 .
A. the partial valence-level orbital diagram for a 4+ ion is . 4s __ 3d 1 1 1 _ _ What is the element from which the ion is derived? write the formula of the oxide this ion forms? B. the partial valence-level orbital diagram for a 1+ ion is . 5s_ 4d 11 11 11 11 11
Transcribed image text: The partial (valence-level) orbital diagram for a 4+ ion is shown below. What is the symbol of the element from which this ion is ...
4 Lecture 2 Pi bond (π): bonding molecular orbital -The bonding electron density lies above and below, or in front and in back of the bonding axis, with no electron directly on the bonding axis, since 2p orbitals do not have any electron density at the nucleus.
3. Draw the partial (valence-level) orbital diagram for each of the following, and write the group number (the "group" is the column the element's in), the penod number (the "period" is the row), and atomic symbol of the element represented. [He]2s22p4 b. c. [Ne]3s23p3 [Ar]4s23d104p4
100% (5 ratings) there are 6 electrons in d orbital of …. View the full answer. Transcribed image text: The partial (valence-level) orbital diagram for a 3+ ion is shown below. Write the symbol of the element from which this ion is derived: Write the formula of the oxide this ion forms:
The Aufbau principle tells you that the lowest-energy orbitals fill first, but the specific order isn't sequential in a way that's easy to memorize. See Resources for a diagram showing the filling order. Note that the n = 1 level only has s orbitals, the n = 2 level only has s and p orbitals, and the n = 3 level only has s, p and d orbitals.
Draw a partial (valence-level) orbital diagram, and write the condensed ground-state electron configuration for each: $$ \begin{array}{llll}{\text { (a) Ti }} & {\text { (b) } \mathrm{Cl}} & {\text { (c) } \mathrm{V}}\end{array} ... Period 4 transition element that forms $3+$ diamagnetic ion (n) Period 4 transition element that forms $2+$ ion ...
The partial (valence-level) orbital diagram for a 2+ ion is shown below. 4d Write the symbol of the element from which this ion is derived. Write the formula of the oxide this ion forms. Question : Be sure to answer all parts.
The partial (valence-level) orbital diagram for a 3+ ion is shown below. __ 4s (no arrows) __ __ __ __ __ 3d (two arrows in the first box, up and down) (One up arrows in the rest of the boxes, 6 arrows total) Write the symbol of the element from which this ion is derived. Write the formula of the oxide this ion forms. The answer is not Fe or Cr.
The partial (valence-level) orbital diagram for a 3+ ion is shown below. tutt 11 (3+ ion) 4s 3d Write the symbol of the element from which this ion is ...
a. Mg 1s22s22p63s13p1 b. Cl 1s22s22p63s23p44s1 c. Mn 1s22s22p63s23p64s23d 44p1 d. Ne 1s22s22p53s1 8.46 Given the following partial (valence‐level) electron configurations, a. identify each element, A Si B F C Sr D S b. rank the four elements in order of increasing atomic size, and F, S, Si, Sr
5.13 The energy level diagram for SH- is shown below. A bond order of 1 is predicted. The S orbital energies are -22.7 eV (3s) and -11.6 eV (3p); the 1s of H has an energy of -13.6 eV. Because of the difference in their atomic orbital energies, the 1s orbital of hydrogen and the
Transcribed image text: The partial (valence-level) orbital diagram for a 3+ ion is shown below. Write the symbol of the element from which this ion is ...
The partial (valence−level) orbital diagram for a 2+ ion is shown below. What is the symbol of the element from which this ion is derived? A. Ti B. Zr C. Cr D. Mo E. Ru Answer: B. This 2+ ion has two electrons in the 4d set. Since the charge of the ion is 2+, two electrons were removed from the atom to make the ion.
0 Response to "37 the partial (valence-level) orbital diagram for a 3+ ion is shown below."
Post a Comment