40 label the energy diagram for a two-step reaction
Draw a potential energy diagram for a two-step endothermic reaction. The first step is the rate determining step. Label all axis and indicate the location of the following: reactants, products, intermediate, activated complex, and transition state. Draw a reaction coordinate diagram for a two-step reaction in which the first step is endergonic, the second step is exergonic, and the overall reaction is endergonic. Label the reactants, products, intermediates, and transition states.
Draw a potential energy (E p ) diagram for a reaction in which ∆H = 80 kJ/mol and E a = +28kJ/mol. Label the axes, activation energy, ∆H, site of the activated complex, reactants and products.
Label the energy diagram for a two-step reaction
Organic Chemistry (9th Edition) Edit edition Solutions for Chapter 6 Problem 21E: Draw an energy diagram for a two-step reaction with Keq > 1. Label the overall ΔG°, transition states, and intermediate. This is an example of a hydrolysis reaction (urea plus water). For each equivalent of carbon dioxide (CO2CO2), two equivalents of ammonia (NH3NH3) are produced. Label the enzyme, substrate, enzyme-substrate complex, enzyme-product complex, and product in the enzymatic reaction for the breakdown of urea by urease using the induced-fit model. is the complex created in the first reaction, while is the activated complex created in the second reaction. Thus, for this two-step process, there are two activated complexes. Example: Draw the potential energy diagram for the following multi-step reaction . Properly label the diagram. Solution: Rate of Reaction is Determined by Slowest Step
Label the energy diagram for a two-step reaction. Draw and label potential energy diagram for the reaction including a molecular structure that could represent an activated complex. The activated complex would show an unstable association of one CH 4(g) molecule and O 2(g) molecule with partial bonds. Check Your Solution The potential energy diagram should match the given information. Label ΔH as positive or negative. Figure shows the energy level diagram for the reaction between methane and oxygen. Based on Figure, the following information can be obtained. (a) The reaction between methane and oxygen to form carbon dioxide and water is an exothermic reaction. (b) During the reaction, the temperature of the mixture increases. Solution for Draw a reaction energy diagram for a two-step exothermic reaction whose second step is faster than the first step. Label the intermediate,… Transcribed image text: Label the energy diagram for a two-step reaction. enthalpy change transition state starting materials RX+H products rate-limiting ...
Transcribed image text: Label the energy diagram for a two-step reaction. R: + HX Energy RX + H RH + X Reaction coordinate Reaction coordinate Answer Bank ... A potential energy diagram plots the change in potential energy that occurs during a chemical reaction. This first video takes you through all the basic parts of the PE diagram. Sometimes a teacher finds it necessary to ask questions about PE diagrams that involve actual Potential Energy values. Question: Label the energy diagram for a two-step reaction. This problem has been solved! See the answer ... Complete Energy Diagram for Two-Step Reaction A Two-Step Reaction Mechanism The transition states are located at energy maxima. The reactive intermediate B+ is located at an energy minimum. Each step has its own delta H and activation energy. The overall energy difference between the starting materials and products is delta H overall.
Using Reaction Diagrams to Compare Catalyzed Reactions The two reaction diagrams here represent the same reaction: one without a catalyst and one with a catalyst. Identify which diagram suggests the presence of a catalyst, and determine the activation energy for the catalyzed reaction: Solution Potential Energy DiagramsPotential Energy Diagrams o Generally, if a reaction requires a low activation energy, it is considered to be a spontaneous reaction (i.e. It is generally a fast reaction) • Reaction Mechanism o Elementary Process • A process in which a reaction occurs in one step (usually occurs between 2 particles) Ternary Process 57CP. Draw an energy diagram for each reaction. Label the axes, the starting material, product, transition state, ΔH°, and E a. a. A concerted, exothermic reaction with a low energy of activation. b. A one-step endothermic reaction with a high energy of activation. c. A two-step reaction. Chemistry questions and answers. Label the energy diagram for a two-step reaction. R. + HX Energy RX + H . RH + X Reaction coordinate Answer Bank enthalpy change intermediates products rate-limiting transition state starting materials activation energy non-limiting transition state.
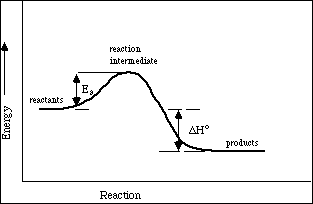
The energy diagram of a two-step reaction is shown below. In the above reaction, a reactant goes through one elementary step with a lower activation energy (transition state 1) to form the intermediate. The intermediate then goes through a second step (transition state 2) with the highest energy barrier to form the product.
Clearly, RX + H. represents the rectants or the starti …. View the full answer. Transcribed image text: Label the energy diagram for a two-step reaction. R. + HX rate-limiting transition state energy intermediates RX + H activation energy non-limiting transition state RH + X: products starting materials enthalpy change reaction coordinate.
Draw an energy diagram for a two-step reaction that is exothermic overall, and consists of a fast but endothermic first step, and a slow but exothermic second step. Indicate DGrxn, as well as DG1* and DG2* for the first and second activation energies, respectively. Label the positions corresponding to the transition states with an asterisk.
The reaction coordinate diagram for the ozone photolysis reaction is a little different from those above because this is an endothermic reaction. Together, the products O 2 and atomic O, have a higher energy than the reactant O 3 and energy must be added to the system for this reaction.
Problem: Label the energy diagram for a two-step reaction. FREE Expert Solution Show answer Answer: 84% (178 ratings) Sign up for free to keep watching this solution Sign up for free. 581,500. students enrolled ... Label the energy diagram for a two-step reaction.
Transcribed image text: Label the energy diagram for a two-step reaction. R: + HX ТЕУ Answer Bank rate-limiting transition state starting materials products ...
So this question wants us to label this energy diagram for this two step reaction, and we want to use all of these terms over here on the ...
A complete enthalpy diagram will include starting energy, ending energy, and E a and delta H. This enthalpy diagram has starting products, ending products, delta H, and activation energy labeled...
Explanation: The figure above represents the reaction profile of a two step, exothermic reaction. The y-axis represents the potential energy of the reaction species, and the x-axis represents the progress of the reaction. The reaction is exothermic because the energies of the products are lower than those of the reactants.
See the answer See the answer done loading. Show transcribed image text. Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 100% (42 ratings) Transcribed image text: Label the energy diagram for a two-step reaction.
Consider the one-step conversion of F to G. Given that the reaction is endothermic by 5 kcal/mol and that the energy difference between G and the transition state for the process is 15 kcal/mol, sketch a reaction-energy diagram for this reaction. Make sure to show how the given energy differences are consistent with your sketch.
Below is an energy diagram illustrating the difference in a catalyzed reaction versus an uncatalyzed reaction. Label the energy diagram and answer the question that follows% (1). Catalyzed reactions have a lower activation energy (rate-limiting free energy of activation) than the corresponding uncatalyzed reaction, resulting in a higher ...
Sketch an energy diagram for a two-step reaction in which both steps are exergonic and in which the second step has a higher-energy transition state than the first. Label the parts of the diagram corresponding to reactant, product, intermediate, overall Δ G †, and overall Δ G ∘. Jil M. Numerade Educator 07:16 Problem 14
Draw a potential energy diagram for a two-step endothermic reaction. The first step is the rate determining step. Label all axis and indicate the location of the following: reactants, products, intermediate, activated complex, and transition state.
19 Mar 2018 — Label the energy diagram for a two-step reaction. ... An energy profile diagram is a theoretical representation that shows how the energy of the ...
is the complex created in the first reaction, while is the activated complex created in the second reaction. Thus, for this two-step process, there are two activated complexes. Example: Draw the potential energy diagram for the following multi-step reaction . Properly label the diagram. Solution: Rate of Reaction is Determined by Slowest Step
This is an example of a hydrolysis reaction (urea plus water). For each equivalent of carbon dioxide (CO2CO2), two equivalents of ammonia (NH3NH3) are produced. Label the enzyme, substrate, enzyme-substrate complex, enzyme-product complex, and product in the enzymatic reaction for the breakdown of urea by urease using the induced-fit model.
Organic Chemistry (9th Edition) Edit edition Solutions for Chapter 6 Problem 21E: Draw an energy diagram for a two-step reaction with Keq > 1. Label the overall ΔG°, transition states, and intermediate.

0 Response to "40 label the energy diagram for a two-step reaction"
Post a Comment