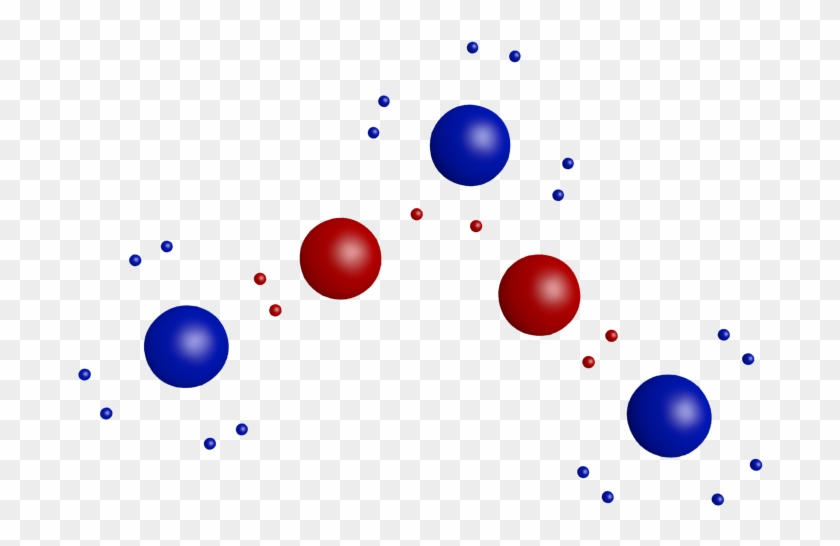
40 lewis dot diagram for aluminum
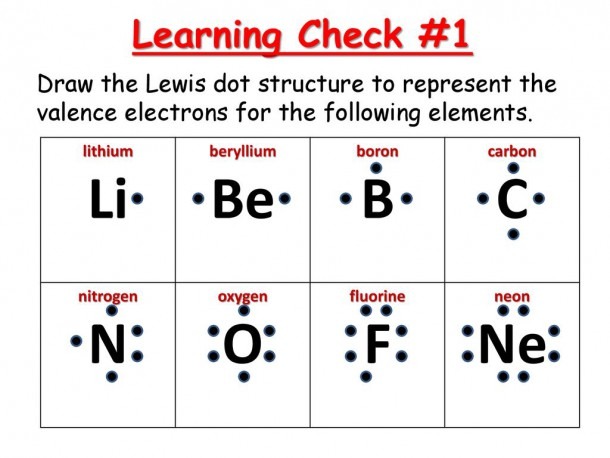
Lewis structures (also known as Lewis dot structures or electron dot structures) are diagrams that represent the valence electrons of atoms within a molecule. Why does aluminum have 3 dots? Answer: Aluminum is in group IIIA of the periodic table therefore it has three valence electrons. In Lewis dot notation aluminum has three dots as it is group 13 and has 3 valence electrons. ... The Lewis dot diagram for neon has a pair of electrons on each side of neon symbol, Ne, for a total ...
Draw a Lewis electron dot diagram for an atom or a monatomic ion. In almost all cases, ... The valence electron configuration for aluminum is 3s 23p 1.

Lewis dot diagram for aluminum
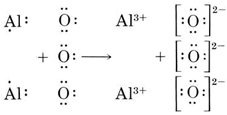
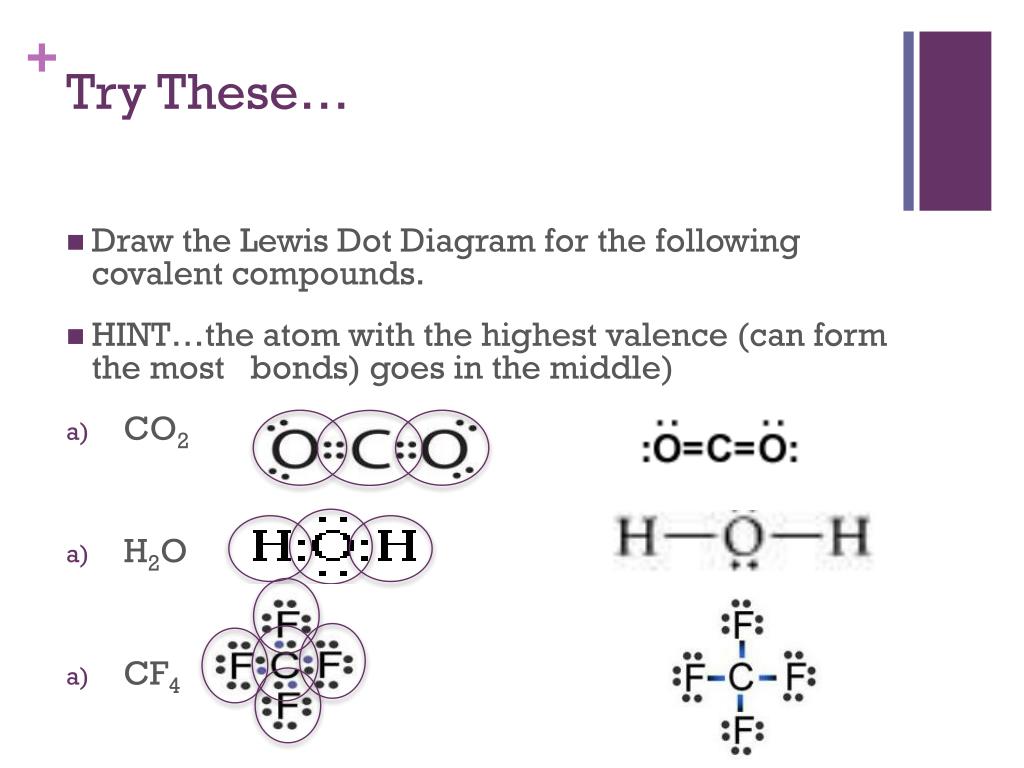
Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond. No Comments on Lewis Structure of Al2O3, Aluminum Oxide Aluminium oxide is a solid ionic compound, made from atoms of one metal (Aluminum) that have lost three electrons each to become +3 cations, and atoms of a non-metal (oxygen) which have gained two electrons each to become -2 anions. Hence, we have to choose the lewis diagram that has the least formal charge on each atom, Therefore, the aluminium central atom is provided with only 6 electrons instead of 8 for completing the octet shell. Summary The total valence electron is available for drawing the Aluminium chloride lewis structure is 24.
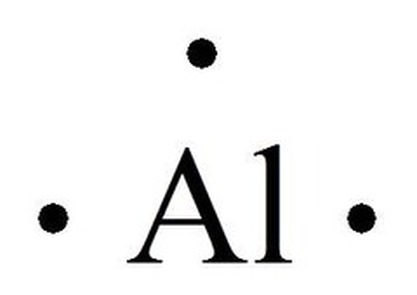
Lewis dot diagram for aluminum. In an electron dot diagram (Lewis Structure) of aluminum. (A1), how many dots should be drawn around the element's symbol? why? What is the Lewis electron dot diagram for each element? aluminum selenium Solution The valence electron configuration for aluminum is 3s23p1. So it would have three dots around the symbol for aluminum, two of them paired to represent the 3selectrons: The valence electron configuration for selenium is 4s24p4. The Lewis dot structure for aluminum includes the symbol, "Al," and a total of three dots around the symbol. a. True. b. False. Lewis Structure: Lewis Dot ...1 answer · Top answer: The given statement is true Aluminum is known to contain 3 valence electrons. As such, we must draw a total of three dots around the chemical symbol... Lewis structures, also known as Lewis dot diagrams, Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDS), are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule.
What is the Lewis electron dot diagram for each element? aluminum selenium Solution The valence electron configuration for aluminum is 3 s2 3 p1. So it would have three dots around the symbol for aluminum, two of them paired to represent the 3 s electrons: The valence electron configuration for selenium is 4 s2 4 p4. Aug 18, 2021 — Answer: Aluminum belongs to group IIIA of the periodic table and therefore has three valence electrons. The symbol of aluminum is Al, which is ... A step-by-step explanation of how to draw the Lewis dot structure for Al (Aluminum). I show you where Aluminum is on the periodic table and how to determine... draw the lewis structure for aluminum sulfide ionic compound and what chemical formula the lewis theory predicts? I do not know what to do with the 6 and 3 dot structure to combine them. Such as the AL2S AL has three dots and S has 6 dots and to balance they share to to make the 8 but I do not know where to go from here.
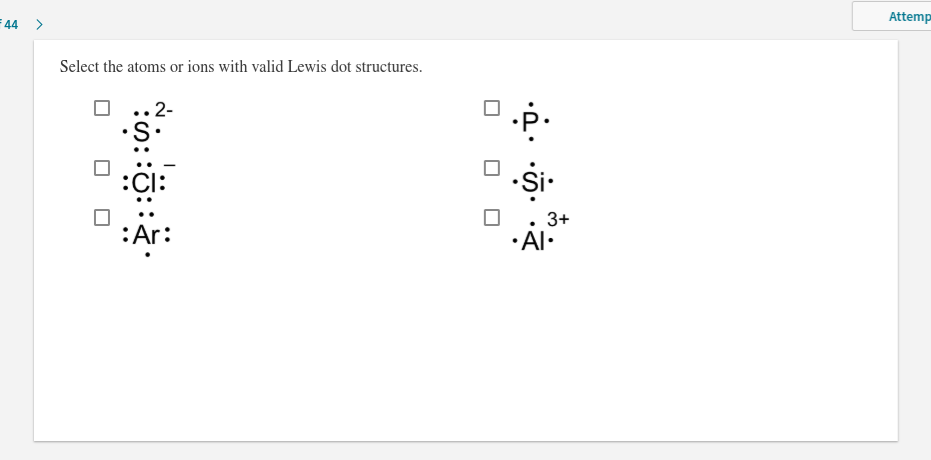
Electron Dot Structures Formula Questions: 1. Give the electron dot structure for aluminum. Answer: Aluminum is in group IIIA of the periodic table therefore it has three valence electrons. The symbol for aluminum is Al which will be surrounded by three dots. 2. Give the electron dot structure of chlorine. Answer: Aluminum / Silicon / Potassium Xenon / Sulfur / Carbon Hydrogen / Helium (watch out!) / Bromine Selenium / Nitrogen / Barium Chlorine / Gallium / Argon. WKS 6.2 - LDS for Ions/ Typical Charges. Determine the common oxidation number (charge) for each of the following ions, and then draw their Lewis Dot Structure. What is the Lewis dot structure for carbon? Lewis Symbols For example, the Lewis symbol of carbon depicts a "C' surrounded by 4 valence electrons because carbon has an electron configuration of 1s 2 2s 2 2p 2. The Lewis symbol for carbon: Each of the four valence electrons is represented as a dot. No, not exactly. It is an ionic compound so it would not have a Lewis dot structure. However, the carbonate anion, CO3^2- does have a Lewis dot structure.
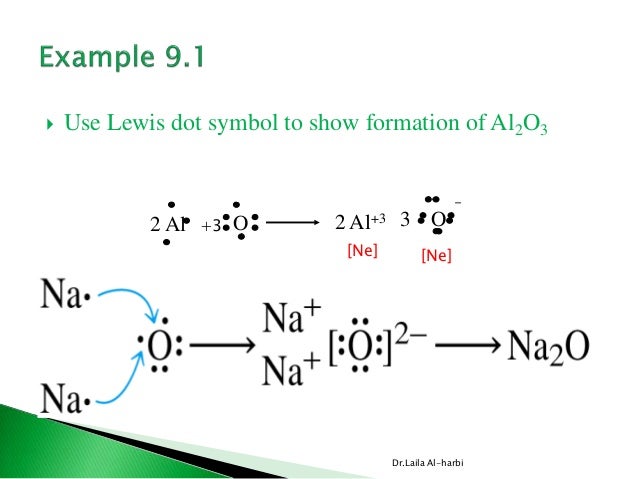
Aluminum has three valence electrons, oxygen has six valence electrons. The formula of aluminum oxide isAl2O3 ...1 answer · Top answer: Hint: We can say that electron dot structure is nothing but a representation of Lewis dot diagram of the valence electrons of an atom that uses dots around ...
Electron Distributions Into Shells for the First Three Periods. A chemical element is identified by the number of protons in its nucleus, and it must collect an equal number of electrons if it is to be electrically neutral.
It is also known as the Lewis dot diagram or electron dot structure. It is the structural illustration of the position of the valence electrons, involved in the formation of a chemical bond, around the atoms inside a molecule. The dots in the proximity of the atoms represent their valence electrons.
Draw the Lewis dot diagram for aluminum. Lewis Dot Diagram: A Lewis dot diagram describes the distribution of all valence electrons present in a covalent molecule or compound, as well as a single...
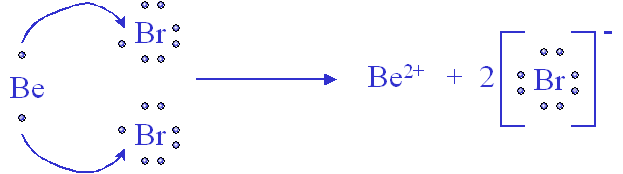
It is a colorless, sublimable hygroscopic solid; hence old samples tend to be hydrated, mostly as aluminium tribromide hexahydrate (AlBr3·6H2O). What is the Lewis dot of aluminum? The Lewis dot structure for aluminum includes the symbol, "Al," and a total of three dots around the symbol. What is the bond between Al and Br? AlBr Bond Polarity
Is it possible to do a lewis dot structure for a metal such as gold or aluminum? For example, would "Al Al" be correct since aluminum is content with 6 valence electrons instead of the usual 8 (octet rule). How would you do gold or copper? My son's teacher has asked him to draw a lewis dot structure for a metal. Thanks!
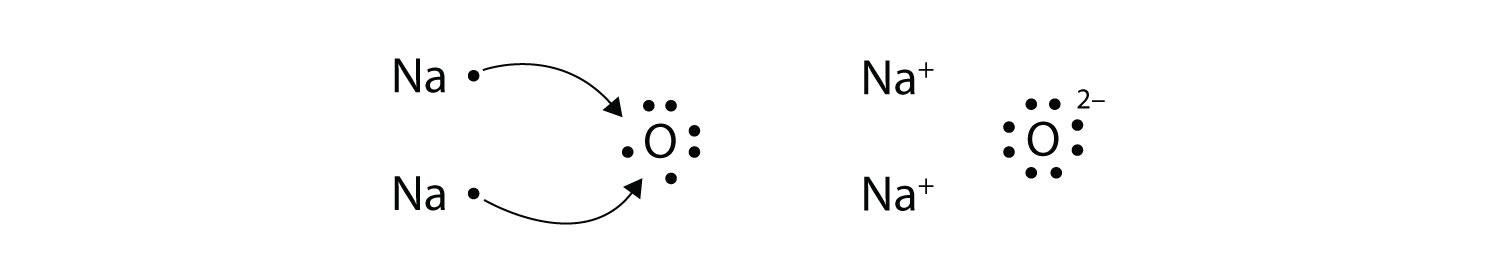
S o l u t i o n According to Figure 9.1, the Lewis dot symbols of Al and O are Because aluminum tends to form the cation (Al 3+) and oxygen the anion (O2−) in ionic compounds, the transfer of electrons is from Al to O. ... = 12 18 lone pairs (18x2) = 36 Total = 48 54 Example 9.9 Draw the Lewis structure for aluminum triiodide (Al I3). AlI 3 ...
Since the ratio of aluminum ions to iodide ions is 1:3, the Lewis structure shows one aluminum ion and three iodide ions. These charges combine to give a net charge of 0. These ions stick together ...
Click to see full answer. Similarly, what is the Lewis dot structure for aluminum? Answer: Aluminum is in group IIIA of the periodic table therefore it has three valence electrons. The symbol for aluminum is Al which will be surrounded by three dots. 2. Secondly, how many valence electrons does aluminum oxide have? 3 valence electrons
A Lewis dot diagram is a representation of an element surrounded by its valence electrons. The diagram consists of the element symbol (from the periodic table), with dots on the top, bottom, and sides representing the sand psub-levels of its valence shell. For example, aluminum has 3 valence electrons. The orbital-notation electron
Electron Dot Diagrams There is another model called the electron dot or Lewis diagram. This system represents an atom and its valence electrons. The electron dot diagram uses the symbol of the element to replace the nucleus and inner shell electrons. The electrons in the valence shell are shown as dots placed around the symbol.
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom.
Mar 24, 2021 — Lewis electron dot diagrams use dots to represent valence electrons around ... So it would have three dots around the symbol for aluminum, ...
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the ...
The oral and ip doses which were lethal in 50% of animals (LD50) were determined for aluminum nitrate (Al (NO3)3), aluminum chloride (AlCl3), aluminum sulfate (Al2 (SO4)3), and aluminum bromide (AlBr3) in Sprague Dawley rats and Swiss mice. Acute oral toxicities of the compounds were much lower than acute ip toxicities.
Oct 26, 2019 · To draw the lewis Dot structure of aluminium (Al), we have to find out the valence electrons of aluminium (Al) first.We express valence electrons as dots in lewis dot structure. To get the valence electrons of aluminium (Al),we need to look at the electronic configuration of aluminium (Al). Al (13)=1s²2s²2p⁶3s² 3p¹.
A step-by-step explanation of how to draw the AlI3 Lewis Dot Structure.For the AlI3 structure use the periodic table to find the total number of valence elec...
Hence, we have to choose the lewis diagram that has the least formal charge on each atom, Therefore, the aluminium central atom is provided with only 6 electrons instead of 8 for completing the octet shell. Summary The total valence electron is available for drawing the Aluminium chloride lewis structure is 24.
No Comments on Lewis Structure of Al2O3, Aluminum Oxide Aluminium oxide is a solid ionic compound, made from atoms of one metal (Aluminum) that have lost three electrons each to become +3 cations, and atoms of a non-metal (oxygen) which have gained two electrons each to become -2 anions.
Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond.









.png)










0 Response to "40 lewis dot diagram for aluminum"
Post a Comment