42 below construct an orbital interaction diagram
Transcribed image text: Below, construct an orbital interaction diagram for molecular orbital formation by dragging the images that represent various ... A schematic molecular orbital diagram for the Fe-Fe interaction in an S = I valence-delocalized Fe Fe pair based on effective C v symmetry at the Fe sites and the observed electronic transitions for the valance-delocalized [Fe2S2l cluster is shown in Fig. 15. The dominant interaction (responsible for the S = ground state) is the a overlap between the pair of orbitals, with progressively ...
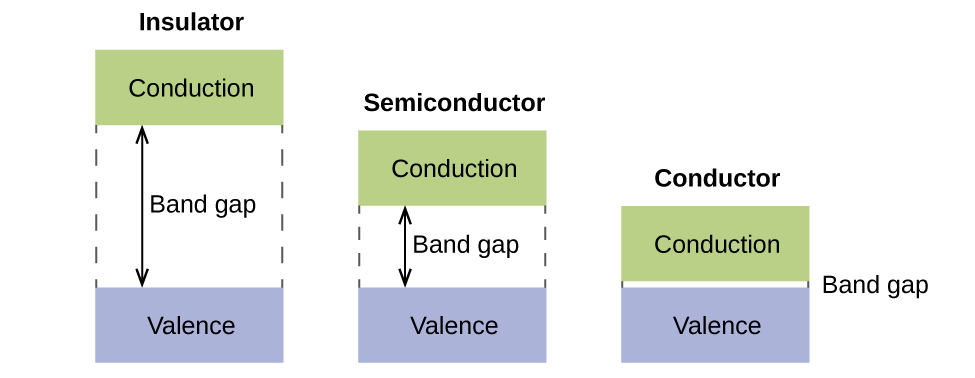
the pi(2p) bonding orbitals are LOWER than the sigma(2p) bonding orbitals.N2(2-) has a bonding order of 2, which predicts that there will be a stable double ...
Below construct an orbital interaction diagram
Transcribed image text: Below, construct an orbital interaction diagram for molecular orbital formation by dragging the image that represents various ... Based on these values, we can construct the orbital interaction energy diagram shown on the right. The diagram highlights the interaction between the HOMO of the diene and the LUMO of the dienophile, which leads to the formation of a new bonding molecular orbital between the two molecules. Pi bond (π): bonding molecular orbital -The bonding electron density lies above and below, or in front and in back of the bonding axis, with no electron directly on the bonding axis, since 2p orbitals do not have any electron density at the nucleus. These are always second or third bonds overlapping a sigma bond formed first.
Below construct an orbital interaction diagram. Orbital Interaction Diagram 1. Plot atomic valence orbital en ergies (or fragment orbitals for More complex molecules). 2. Determine which orbitals can interact (those with S 0). 3. Determine magnitude of each interaction: scales directly with magnitude of overlap scales inversely with orbital energy difference 4. Plot MO energies and draw orbitals Diagram for A. 2 (A = Li, Be) Li. 2. Only two valence electrons, i.e. σ. s 2. σ * s 0. Bond order =1. Li. 2 . exists in gas phase over metallic lithium. Diamagnetic "Be. 2 " σs. 2. σ * s. 2 . Bond order = 0 - no net bonding energy, so molecule does not exist. Beryllium in gas phase is monatomic. Use Aufbau, Pauli, Hund - just as in filling atomic orbitals Below, construct an orbital interaction diagram for molecular orbital formation by dragging the images that represent various orbital types (e.g., atomic, bonding, antibonding) into the relevant boxes. Identify the number of nodes in each atomic and molecular orbital. The diagram shows how the molecular orbitals in lithium hydride can be related to the atomic orbitals of the parent atoms. One thing that makes this diagram look different from the ones we have seen previously is that the parent atomic orbitals have widely differing energies; the greater nuclear charge of lithium reduces the energy of its 1 s orbital to a value well below that of the 1 s hydrogen orbital.
Transcribed image text: Below, construct an orbital interaction diagram for molecular orbital formation by dragging the images that represent various ... The valence molecular orbital gram for planar nitryl fluorideenergy level dia (𝑁𝑁2𝐹𝐹 𝐶𝐶with nitrogen as the central atom) is shown below. Note that the or π character of each MO is σ indicated, but NOT the nature of the overall interaction (i.e. whether it has bonding, antibonding, or non-bonding character). with eg orbital set of metal ions and produces doubly-degenerate bonding (eg) and doubly-degenerate antibonding molecular orbital (e* g) set. The t2g orbital set of the metal center remains non-bonding in nature. The electron-filling in various orbitals is done in accordance with the Aufbau principle, Hund's rule and Pauli exclusion principle. Transcribed image text: Below, construct an orbital interaction diagram for molecular orbital formation by dragging the images that represent various ...
We assume that orbital order is the same as that for N2. The bond order is Figure The molecular orbital energy-level diagram for both the NO+ and CN-ions. Figure A partial molecular orbital energy-level diagram for the HF molecule. This interaction introduces an element of s-p mixing, or hybridization, into the molecular orbital theory. The interaction of the two bonded atoms with the bonding electrons produces a more stable arrangement for the atoms than when separated. Electrons usually occupy these orbitals. A sigma bonds is always the first bond formed between two atoms. Sigma star (σ*) antibonding molecular orbital – Normally this orbital is empty, but if it should Molecular Orbital Energy Level Diagrams: Homonuclear Diatomics . This exercise assumes that you are familiar with the "count and sort" algorithm described n i Exercise 2.3. The table of atomic orbital energies is repeated below for easy reference. Table of Atomic Orbital Energies. All Energies are in Ry. 1s 2s 2p H -1.00 He -1.81 In picture 1 we show the molecular orbital structure of F2. In picture 2 we show the overlapping p orbitals, which form the bond between the two fl uorine atoms, in red and green gradients. The dashed lines show the remaining p orbitals which do not take part in the bonding. σ z y x σ* x y z Construct the molecular orbital diagram for ...
Below, construct an orbital interaction diagram for molecular orbital formation Question: Consider two 2p orbitals, one on each of two different atoms, oriented side-to-side, as in the figure. Images bringing these nuclei together so that overlap occurs as shown in the figure. This overlap results in a system of molecular orbitals.
Two p-atomic orbitals (one from each nitrogen) atom combine to form two molecular orbitals, the bonding molecular orbital σ2px and antibonding molecular orbital σ*2px. The other four p-atomic orbitals (two from each nitrogen) atom combine to give four molecular orbitals, two bonding molecular orbitals i.e. π2py and π2pz, while two antibonding molecular orbitals i.e. π*2py and π*2pz.
An orbital is characterized by a size, shape, and orientation in space below, construct an orbital interaction diagram for molecular Below, construct an orbital interaction diagram for molecular orbital formation by dragging the image that represents various orbital types (e.g., atomic, bonding, antibonding) into the relevant boxes.
#1. Draw the MO diagram for `B_2`. First step is to determine which MO diagram we're using. In this case, we're using the standard one. Draw out the MO diagram and label in the valence electrons. Boron has 2 electrons in the `2s` orbitals and 1 electron in the `2p` orbital. That's it for the MO diagram of `B_2`!
Question: Below, construct an orbital interaction diagram for molecular orbital formation by dragging the images that represent various orbital types (e.g., ...
Solved A Below Construct An Orbital Interaction Diagram Chegg. Solved the molecular orbital diagram for the fluorine mol chegg com - um, anti bonding divide by two. what is the molecular orbital diagram of o2 and f2 quora - on the atomic level, bond order is the number of bonded electron pairs between two atoms. molecular orbital theory .
Bond order: (1/2)*(#el. in bonding MOs - #el. in antibonding MOs) Note: Use MO Diagram to determine # of electrons in bonding and antibonding orbitals. Below is shown the atomic orbitals that may be used to construct molecular orbitals for carbon monoxide (CO).
Construct a "molecular orbital diagram" of the kind shown in this lesson for a simple diatomic molecule, and indicate whether the molecule or its positive and negative ions should be stable. A molecule in which all the electrons are paired, is called diamagnetic while molecule which has one or more unpaired electron is called paramagnetic.
a) Below, construct an orbital interaction diagram for molecular orbital formation by dragging the images that represent various orbits types (e.g., atomic, bounding, antibonding) into the relevant boxes. b) Identify the number of nodes in each atomic and molecular orbital.
Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics.
Writing half-equations Describing entropy Oxidation state and formal charge Lewis structures and resonance Structure and bonding Part 1: Historical background Structure and bonding Part 2: Atomic spectroscopy Structure and bonding Part 3: Atomic orbitals Structure and bonding Part 4: Interaction between orbitals from different atoms Structure and bonding Part 5: Interaction between orbitals of the same atom Resonance and curly arrows Elementary reactions and reaction mechanisms ...
Question 7 of 16 Incorrect Map Sapling Learning macmillan learning Construct the.Molecular Orbital Diagrams of Diatomic Molecules Introduction: In chemistry molecular orbital (MO) theory is a method for determining molecular structure in which electrons are not assigned to individual bonds between atoms, but are treated as moving under the influence of the nuclei in the whole molecule.
1) Predict the product(s) and draw the mechanism 2) Use Frost"$ for when the compound below are treated with circle to construct orbital energy diagram for cyclopentadienyl anion, excess chloroethane and AICI3 (1.5 points) Clearly draw the orbital energies diagram with the correct the number of electron in each Ievel, label itas aromatic Q antiaromatic_according t0 Huckels rule .
got a question telling us to produce an M O diagram. A molecular little diagram for F two plus and status box. So first things first. Let's figure out how many electrons we need to use Flooring has seven, but we've got two of them and we've got a positive charge. Meaning we lose one of those electrons seven times to this 14 minus one is 13 electrons.
equivalent sp2 orbitals, leaving one p orbital untouched. The process is shown below. 2s 2p X 2p y 2p z Potential energy sp2 hybridization sp2 sp2 sp2 p In this top view, the unhybridized p orbital cannot be seen because it also arranges itself to be as far apart from the sp2 orbitals as possible.
Below, construct an orbital interaction diagram for molecular orbital formation by dragging the image that represents various orbital types (e.g., atomic, bonding, antibonding) into the relevant boxes. b) Identify the number of nodes in each atomic and molecular orbital.
It is possible for the 2s orbital on one atom to interact with the 2p z orbital on the other. This interaction introduces an element of s-p mixing, or hybridization, into the molecular orbital theory. The result is a slight change in the relative energies of the molecular orbitals, to give the diagram shown in the figure below.
The orbital energies are summarized in an orbital interaction diagram, shown in Fig. 1.14. This diagram is a plot of orbital energy versus the position of the two interact-ing nuclei. The isolated atomic orbitals and their energies are shown on the left and right sides of the diagram, and the molecular orbitals and their energies are shown in the cen-
Below, construct an orbital interaction diagram for molecular orbital formation by dragging the images that represent various orbital types (e.g., atomic, bonding, antibonding) into the relevant boxes. Identify the number of nodes in each atomic and molecular orbital. When two electrons occupy the bonding molecular orbital above, what type of bond results?
Below, construct an orbital interaction diagram for molecular orbital formation by dragging the images that represent various orbital types (e.g., atomic, bonding, antibonding) into the relevant boxes. Identify the number of nodes in each atomic and molecular orbital. When two electrons occupy the bonding molecular orbital above, what type of bond results?
Pi bond (π): bonding molecular orbital -The bonding electron density lies above and below, or in front and in back of the bonding axis, with no electron directly on the bonding axis, since 2p orbitals do not have any electron density at the nucleus. These are always second or third bonds overlapping a sigma bond formed first.
Based on these values, we can construct the orbital interaction energy diagram shown on the right. The diagram highlights the interaction between the HOMO of the diene and the LUMO of the dienophile, which leads to the formation of a new bonding molecular orbital between the two molecules.
Transcribed image text: Below, construct an orbital interaction diagram for molecular orbital formation by dragging the image that represents various ...

0 Response to "42 below construct an orbital interaction diagram"
Post a Comment