42 how to make a bohr diagram
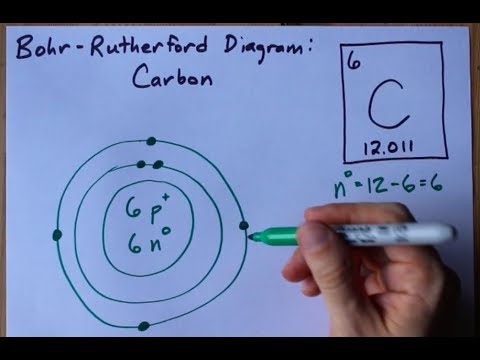
7. Bohr Diagrams 1) Draw a nucleus with the element symbol inside. 2) Carbon is in the 2nd C period, so it has two energy levels, ... Drawing Bohr-Rutherford diagrams is super easy using the following steps: Find the number of protons, neutrons and electrons for the atom. The number of protons is the atomic number. The number of neutrons can be found by subtracting the number of protons from the atomic mass rounded to the nearest whole.
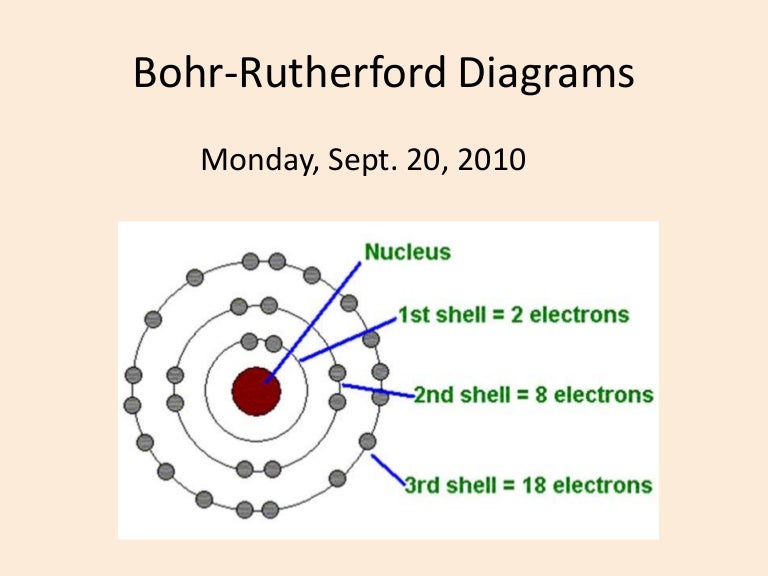
Bohr diagrams show electrons orbiting the nucleus of an atom In the Bohr model, electrons are pictured as traveling in circles at different shells, Each element, when electrically neutral, has a number of electrons For example, the 1n shell represents the first energy level located closest to the nucleus.Now offering rare physics books for sale ...
How to make a bohr diagram
Steps to draw the Bohr Model of Carbon atom · 1. Find the number of protons, electrons, and neutrons in the Carbon atom · 2. Draw the nucleus of an atom · 3. Draw ...Total valence electrons in Carbon: 4Electrons in the Second shell(L): 4Number of electrons: 6Number of protons: 6 A step by step program for creating Bohr Diagrams and Electron dot structures. Usefule for Index Cards and Flash cards for chemistry and physical science stude… SlideShare uses cookies to improve functionality and performance, and to provide you with relevant advertising. Bohr Diagram: The First Element In order to make a Bohr diagram, you need to know the number of protons, neutrons, and electrons the element has. In this section, we'll show a sample Bohr diagram for hydrogen. H —Hydrogen 1 proton 1 electron 0 neutrons
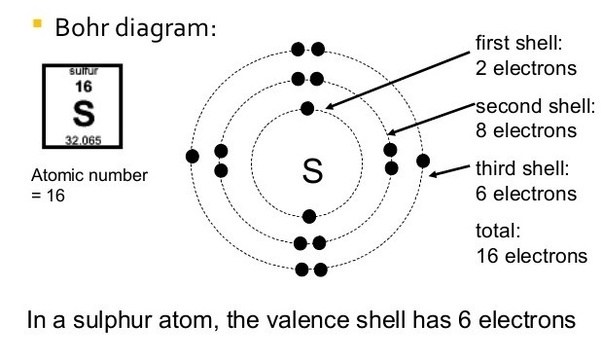
How to make a bohr diagram. The Bohr Atom is a very simplified model of the electron positions of each element of the Periodic Table. Each Row of the periodic table is represented by an orbit. Hydrogen and Helium are in the first energy level (row) of the periodic table and their Bohr Models would have one orbit. The elements of the second energy level (row) Lithium through neon have two orbits in their Bohr Model. Drawing Bohr Models Draw the nucleus Write the number of neutrons and the number of protons in the nucleus. Draw the first energy level Draw the electrons in the energy levels according to the rules below. Make sure you draw the electrons in pairs Keep track of how many electrons are put in each level and the number of electrons left to use. 15 Aug 2020 — The Bohr model shows the atom as a central nucleus containing protons and neutrons with the electrons in circular orbitals at specific ... Bohr Diagrams 1) Draw a nucleus with the element symbol inside. C 2)Write the number of Protons and Neutrons the element has inside the nucleus. Put a + by the P and a little 0 by the N. P+ = 6 N0 = 6 Note: Round mass to nearest 1 when figuring neutrons. 5. The electron shells surrounding the nucleus each hold a particular number of electrons.
Drawing Bohr-Rutherford diagrams is super easy using the following steps: Find the number of protons, neutrons and electrons for the atom. The number of protons is the atomic number. Set up the diagram. To set up the diagram, you will need a circle in the middle. Add in orbitals and electrons. What group is CA? Mr. Primmer Demonstrates How to Draw Bohr Rutherford Diagrams! Bohr Diagrams • Find out which period (row) your element is in. • Elements in the 1 st period have one energy level. • Elements in the 2 nd period have two energy levels, and so on. www. chem 4 kids. com . Bohr Diagrams C 1) Draw a nucleus with the element symbol inside. 2) Carbon is in the 2 nd period, so it has two energy levels, or shells. Use the information provided for each element to draw Bohr Model diagrams. Label how many of each there are in the nucleus (e.g. He: 2p, 2n). Then, draw the individual electrons on the appropriate energy levels (keep in mind the maximum number of electrons allowed on each level - 2, 8, 8, etc. 1. Beryllium - P. E. N 2.
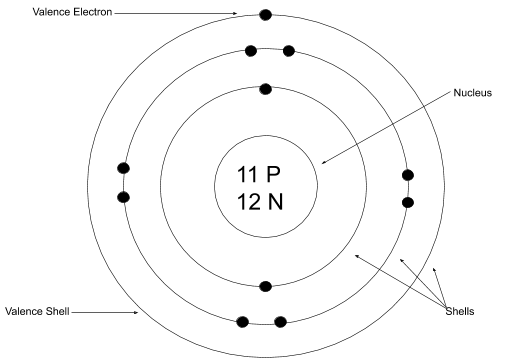
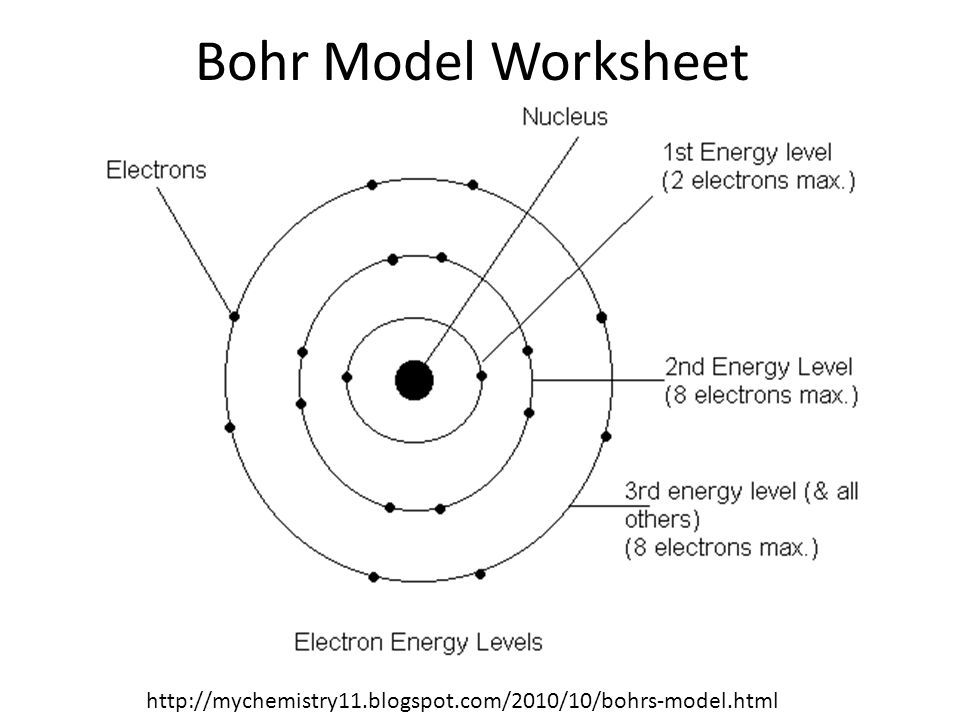
Valence Electrons & Bohr Diagrams Atomic Structure Atoms have a nucleus that contains Protons and Neutrons Electrons are contained in shells that surround the nucleus An atom is made of mostly empty space Protons have a positive charge Electrons have a negative charge Neutrons are Neutral Valence Electrons Each electron shell can hold a certain number of electrons Electron shells are filled ... Bohr Diagrams 1) Check your work. 2) You should have 6 total electrons for Carbon. 3) Only two electrons can fit in the 1st shell. 4) The 2nd shell can hold up to 8 electrons. 5) The 3rd shell can hold 18, but the elements in the first few periods only use 8 electrons. 6p 6n. Bohr Diagrams Try the following elements one at a time: a) H b) He Bohr Diagram Practice Worksheet - Addressing troubles is a wonderful method to aid students boost their algebra abilities. Recognize an unidentified worth in a number sentence with an equal sign. The student must find out the worth of a number sentence utilizing fundamental math procedures. Answer: Bohr models show energy levels of electrons. Each orbital represents an energy level. The first level contains up to 2 electrons, the second contains up to 8, the third contains up to 18 , and this number tends to continue to increase. Now they don't contain over 8 until you get to the ...
To draw a Bohr model of an atom, first find the number of protons, neutrons and electrons in the atom from its atomic weight and atomic number. After that, place the neutrons and the protons in the nucleus, and draw the electrons in their designated shells. Find the number of electrons, protons and neutrons

MISS SWISS helps makeup wearers with glamour on the go no matter where their travels take them. The MISS SWISS makeup case was created from a personal purse catastrophe. It helps you apply your makeup easily on the go, prevents your makeup from spilling, and to prevent your makeup from getting lost. It is the perfect something blue for your wedding day. Touching up your makeup after your ceremony or between photos is a breeze.
The Bohr Model of Beryllium(Be) has a nucleus that contains 5 neutrons and 4 protons. This nucleus is surrounded by two-electron shells named K-shell and L-shell. The outermost shell in the Bohr diagram of Beryllium contains 2 electrons that also called valence electrons.
How do you create a Bohr Diagram? Well, first you must know how many protons, electrons, and neutrons the element has. The way you are able to figure out that piece of information is to first locate the atomic number and atomic mass. By just looking at the atomic number, right off the bat we know that the number of protons is the same as the ...
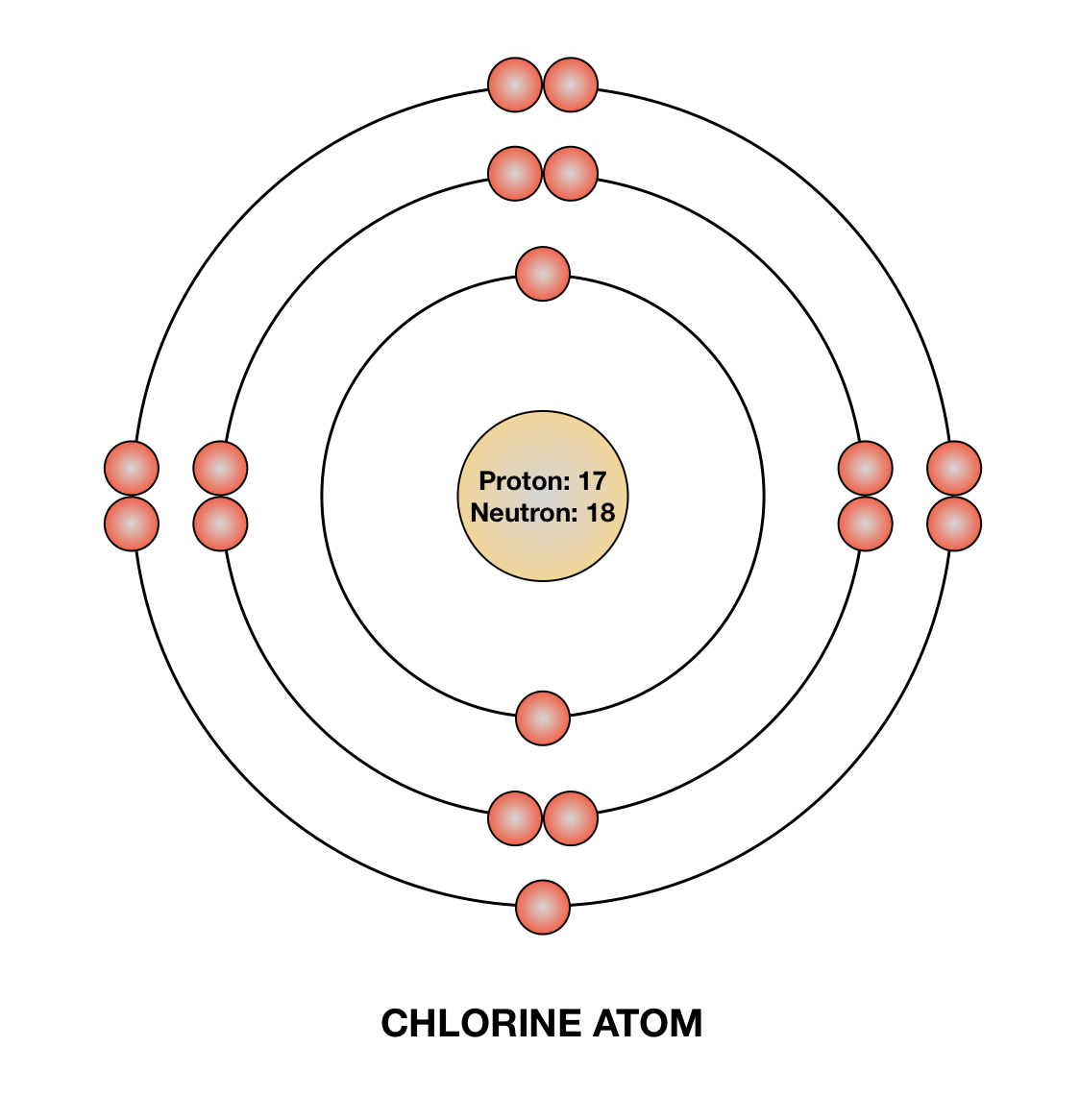
How to draw the Bohr-Rutherford Diagram for Phosphorous. 2 electrons can go in the first shell, 8 in the second, 8 in the third, and so on...
Jul 05, 2016 · How to draw Bohr Diagrams – a step by step tutorial. I updated the Google Slides and worksheet for my lesson on drawing Bohr Diagrams. This lesson will walk your students through the basics on how to draw a Bohr Diagram for the first 20 elements on the periodic table. I also created a simple worksheet for students to record their drawings and ...
The Bohr Model of Argon(Ar) has a nucleus that contains 22 neutrons and 18 protons. This nucleus is surrounded by three-electron shells named K-shell, L-shell, and M-shell. The outermost shell in the Bohr diagram of Argon contains 8 electrons that also called valence electrons.
25 Jul 2021 — Of these two models, the Bohr model is simpler and relatively easy to understand. A model is useful because it helps you understand what's ...
Bohr Diagrams 1) Draw a nucleus with the element symbol inside. 2) Carbon is in the 2nd C period, so it has two energy levels, or shells. 3) Draw the shells around the nucleus. 5. Bohr Diagrams 1) Add the electrons. 2) Carbon has 6 electrons. C 3) The first shell can only hold 2 electrons. 6.
How to draw the Bohr-Rutherford (Shell) Diagram for Selenium. This video explains how orbital diagrams are used to fill larger shell models for 20 or more e...
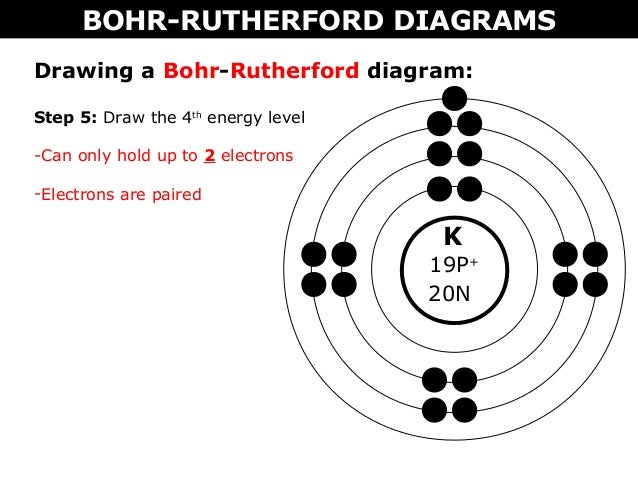
How to draw the Bohr-Rutherford Diagram for Potassium. 2 electrons can go in the first shell, 8 in the second, 8 in the third, and so on...
24 Apr 2017 — A Bohr diagram is a simplified visual representation of an atom that was developed by Danish physicist Niels Bohr in 1913.
Bohr model of Helium (He) 2: 3: Bohr model of Lithium (Li) 2, 1: 4: Bohr model of Beryllium (Be) 2, 2: 5: Bohr model of Boron (B) 2, 3: 6: Bohr model of Carbon (C) 2, 4: 7: Bohr model of Nitrogen (N) 2, 5: 8: Bohr model of Oxygen (O) 2, 6: 9: Bohr model of Fluorine (F) 2, 7: 10: Bohr model of Neon (Ne) 2, 8: 11: Bohr model of Sodium (Na) 2, 8 ...
Calcium has 2 electrons in its first shell, 8 in its second, 8 in its third, and 2 in its fourth.Check me out: http://www.chemistnate.com
Bohr-Rutherford Diagrams of Ions ... Try to make a Bohr-Rutherford ion for phosphorous. 31 P 15 3-Metals will often form bonds with non metals. This is the Basis for Ionic compounds
To draw the Bohr model diagram for any atom, first find the number of protons, electrons, and neutrons of the atom, then, draw the nucleus and write proton and neutrons at the center of it, after that, draw the first electron shell, second electron shell, third electron shell, etc. depending on the availability of electrons.
Bohr Diagram: The First Element In order to make a Bohr diagram, you need to know the number of protons, neutrons, and electrons the element has. In this section, we'll show a sample Bohr diagram for hydrogen. H —Hydrogen 1 proton 1 electron 0 neutrons
A step by step program for creating Bohr Diagrams and Electron dot structures. Usefule for Index Cards and Flash cards for chemistry and physical science stude… SlideShare uses cookies to improve functionality and performance, and to provide you with relevant advertising.
Steps to draw the Bohr Model of Carbon atom · 1. Find the number of protons, electrons, and neutrons in the Carbon atom · 2. Draw the nucleus of an atom · 3. Draw ...Total valence electrons in Carbon: 4Electrons in the Second shell(L): 4Number of electrons: 6Number of protons: 6
:max_bytes(150000):strip_icc()/Bohr-58e690203df78c51620ff02e.jpg)
























0 Response to "42 how to make a bohr diagram"
Post a Comment