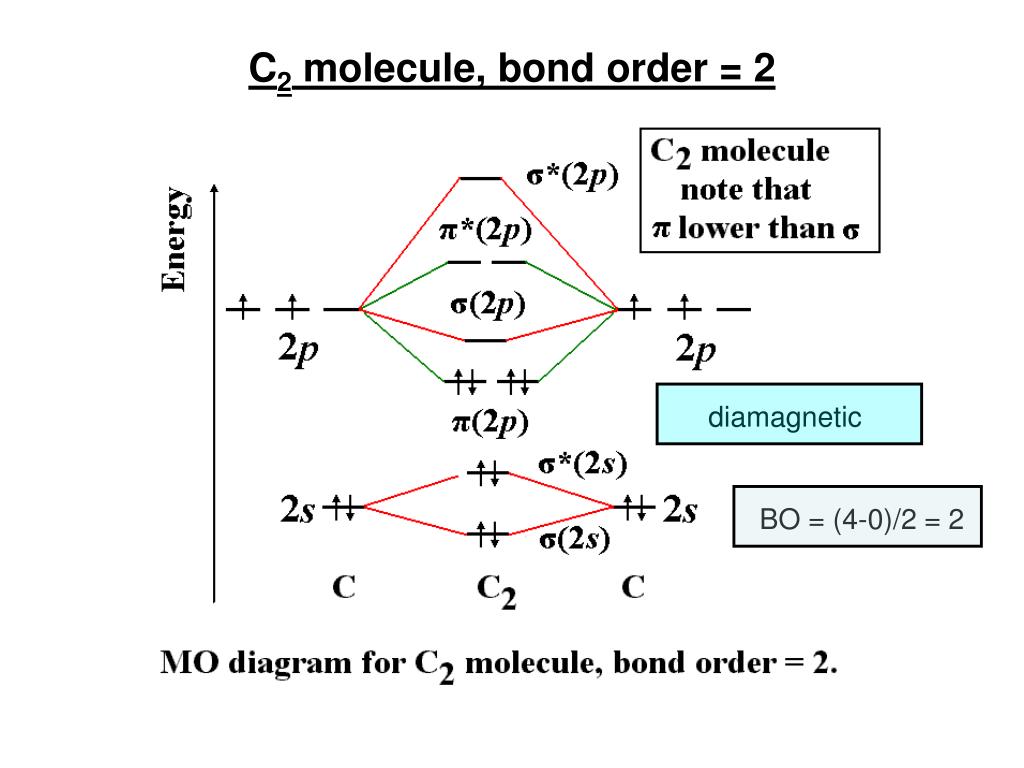
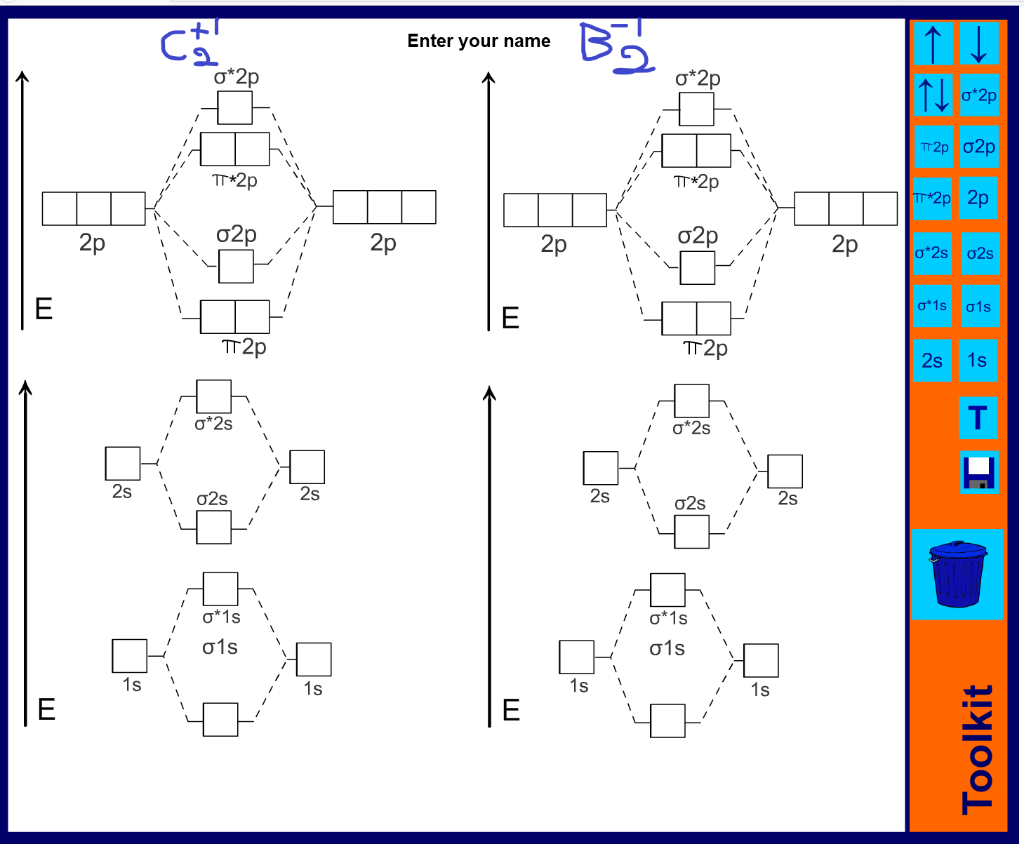
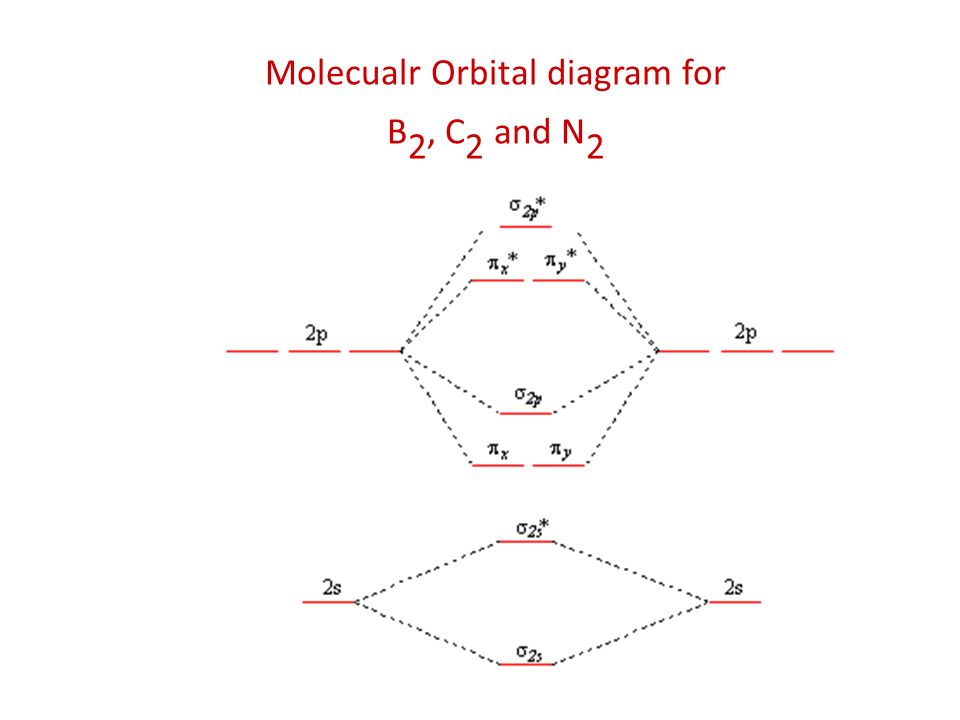
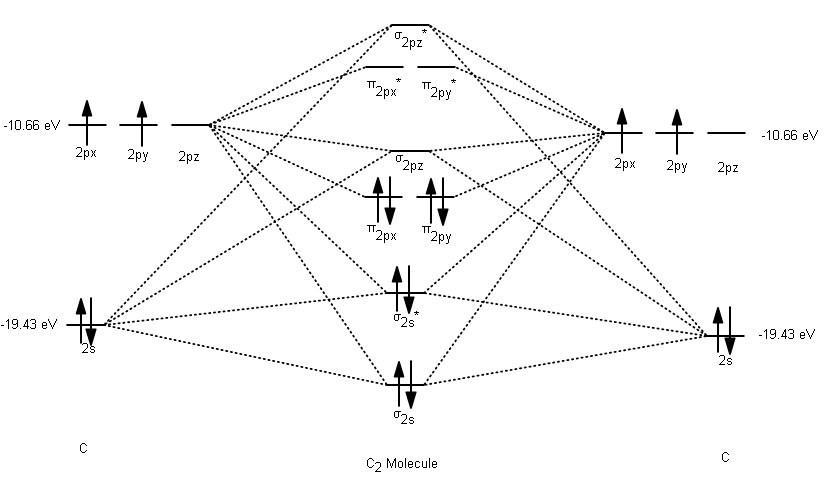
38 mo diagram for c2
Model–view–controller (MVC) is a software design pattern commonly used for developing user interfaces that divide the related program logic into three interconnected elements. This is done to separate internal representations of information from the ways information is presented to and accepted from the user. Draw the MO diagram for acetylide ion C2^2- and calculate its bond order. ... asked Dec 18, 2020 in Chemical Bonding by Aashi01 (13.0k points) closed Dec 18, 2020 by Aashi01. Draw the MO diagram for acetylide ion C 2 2-and calculate its bond order. chemical bonding; class-11; Share It On Facebook Twitter Email. 1 Answer +1 vote ...
When two carbons atoms bond, the pi(2p) bonding molecular orbitals are lower in energy than the sigma(2p) bonding orbitals.C2(2-) has a bond order of 3, so i...

Mo diagram for c2
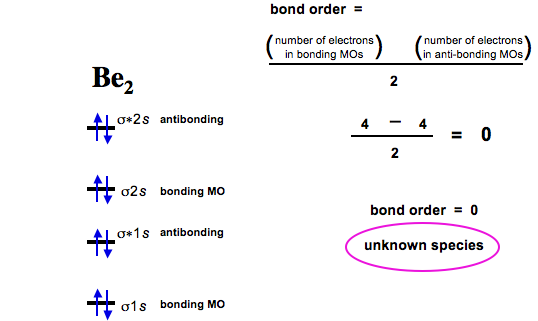
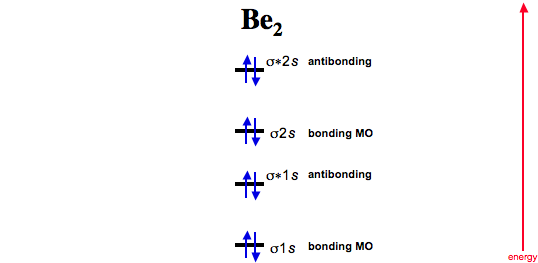
MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H–F nb σ σ* Energy H –13.6 eV 1s F –18.6 eV –40.2 eV 2s 2p So H–F has one σ bond and three lone electron pairs on fluorine Solution for Use MO diagrams to place C2-, C2, and C2- in order of (a) increasing bond energy; (b) increasing bond length. The Weak Force. One of the four fundamental forces, the weak interaction involves the exchange of the intermediate vector bosons, the W and the Z.Since the mass of these particles is on the order of 80 GeV, the uncertainty principle dictates a range of about 10-18 meters which is about 0.1% of the diameter of a proton.. The weak interaction changes one flavor of quark into …
Mo diagram for c2. Q. Using the molecular orbital model, write electronic configurations for the following diatomic species and calculate the bond orders.a. COb. CO+c. CO2+ ... Our tutors rated the difficulty of Use MO diagram to place C2-,C2 and C2+ in order of decreasin... as medium difficulty. Apr 20, 2015 · circuit diagram help from this book. Give your feedback by mailing me. ... Digital Soil Mo isture Tester 14. 22. ... For low fre quency value of capacitor C1 and C2 must be . The link below shows the MO diagram for C2, a species of carbon that has been identified in the gas phase. The electrons in the bonding orbitals are identified as being in two pi bonding orbitals giving that molecule a bond order of 2. The next higher orbital is a sigma bonding orbital. 42.7 g/100 mL (0 °C) 49 g/100 mL (10 °C) 59.8 g/100 mL (25 °C) 71.8 g/100 mL (40 °C) 119.5 g/100 mL (80 °C) 300 g/100 g (120 °C) Solubility: soluble in alcohol, ethyl acetate: Solubility in acetone: 137 g/100 g: Solubility in alcohol (chemistry): 1.82 g/g (0 °C, in CH 3 OH) 1.52 g/g (0 °C, in C 2 H 5 OH) 1.05 g/g (25 °C, in C 3 H 7 OH) 0.793 g/g (0 °C, in C 4 H 9 OH) ...
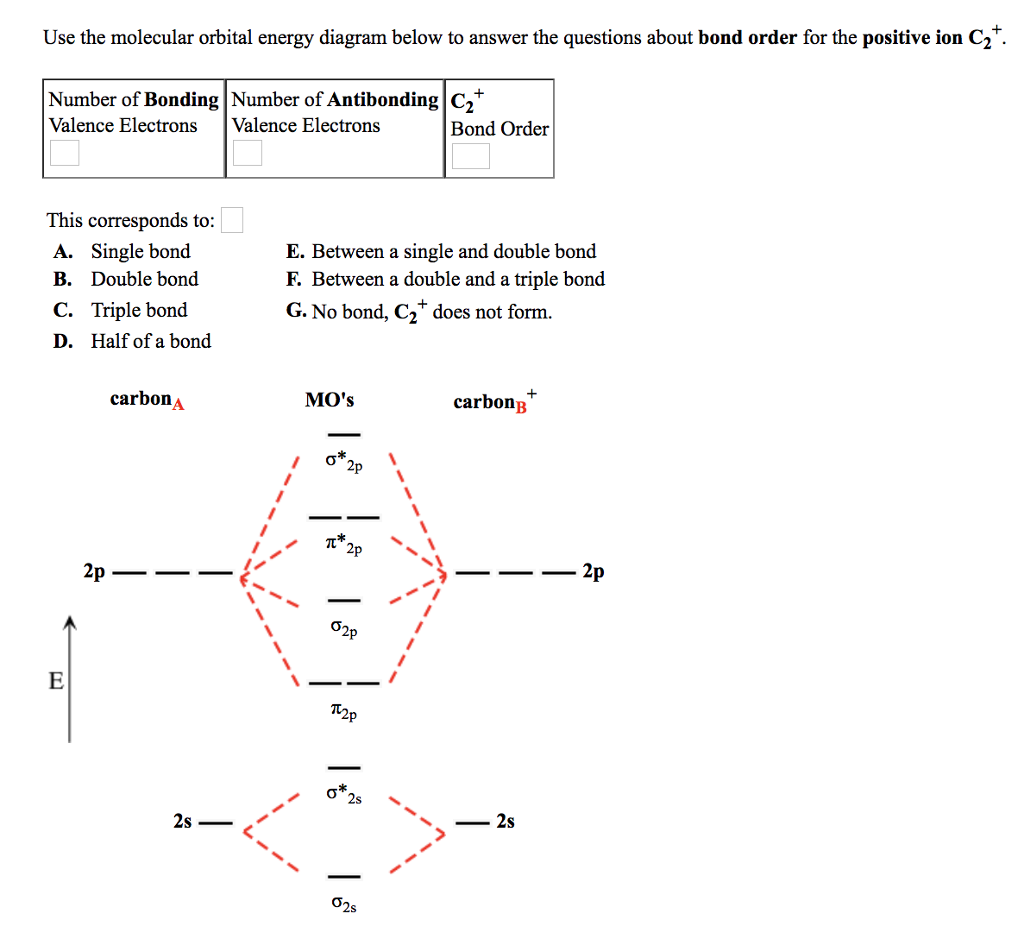
O2 is alpha oxygen-like structured and crystallizes in the monoclinic C2/m space group. The structure is zero-dimensional and consists of eight hydrogen peroxide molecules. O is bonded in a single-bond geometry to one O atom. The O–O bond length is 1.23 Å. Looking at our diagram above, we must be careful not to "double add" the intersection. The easiest way to do this is to take the intersection and add to it the number of CS-only and math-only students: 600 + 200 + 400 = 1200. This number represents the total number of students that have either a math or CS major (that is, the number of students ... Jul 13, 2019 · ① Input Filter Circuit: The double-type filter network, consisting of C1, L1, C2 and C3, primarily suppresses the electromagnetic noise and clutter signals of the input power source to prevent interference to the power supply, as well as high-frequency clutter generated by the power supply itself from interfering with the power grid. Answer to Solved Q1 : 1. Construct an MO diagram of C2- 2. Is C2-an sp 2 orbital from the carbon overlapping with a 2p orbital from each F atom to form sigma bonds, while another sp 2 hybrid orbital forms a sigma bond by overlaping with a sp 2 orbital for oxygen to form a sigma bond. A Pi bond is formed between C and O by the overlap between partially filled p orbitals overlapping between both ...
Al2O3 crystallizes in the monoclinic C2/m space group. The structure is three-dimensional. there are two inequivalent Al3+ sites. In the first Al3+ site, Al3+ is bonded to four O2- atoms to form AlO4 tetrahedra that share corners with seven equivalent AlO6 octahedra and corners with two equivalent AlO4 tetrahedra. The corner-sharing octahedra tilt angles range from 56–63°. Multiple Feedback Band-pass Filter Design Tool. This page is a web application that design a multiple feedback band-pass filter. Use this utility to simulate the Transfer Function for filters at a given frequency, damping ratio ζ, Q or values of R and C. -35-(b) The P.E.S. results are consistent with the MO scheme. Only the Bn(x) MO is strictly nonbonding, while the F(z) MO is weakly bonding, as indicated by the vibrational fine structureon its P.E.S. band. (c) Rather than two lone pairs in approximately sp3 hybrids, the MO scheme suggests a single region of electron density protruding from the back side of the molecule. Chapter 1: Molecular Orbital Concepts A. Concepts of MO Theory. 1. Strong Covalent Bonds. ... An energy level diagram of these orbitals and their energy is illustrated below. q Since both parameters are negative ... q The BMO has a larger coefficient at C2 (the central carbon) than at C1 and C3, corresponding to a larger electron density at C2 ...
The electron configuration of the neutral C2 molecule is -- I'll use the notation given to you in the diagram. C2:(1sσ)2(1s* σ)2(2sσ)2(2s* σ)2(2pπ)4. The electron configuration of the C− 2 ion will be. C− 2:(1sσ)2(1s* σ)2(2sσ)2(2s* σ)2(2pπ)4(2pσ)1. Notice that because the extra electron is added to a bonding MO, the bond order of ...
The Weak Force. One of the four fundamental forces, the weak interaction involves the exchange of the intermediate vector bosons, the W and the Z.Since the mass of these particles is on the order of 80 GeV, the uncertainty principle dictates a range of about 10-18 meters which is about 0.1% of the diameter of a proton.. The weak interaction changes one flavor of quark into …
Solution for Use MO diagrams to place C2-, C2, and C2- in order of (a) increasing bond energy; (b) increasing bond length.
MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H–F nb σ σ* Energy H –13.6 eV 1s F –18.6 eV –40.2 eV 2s 2p So H–F has one σ bond and three lone electron pairs on fluorine


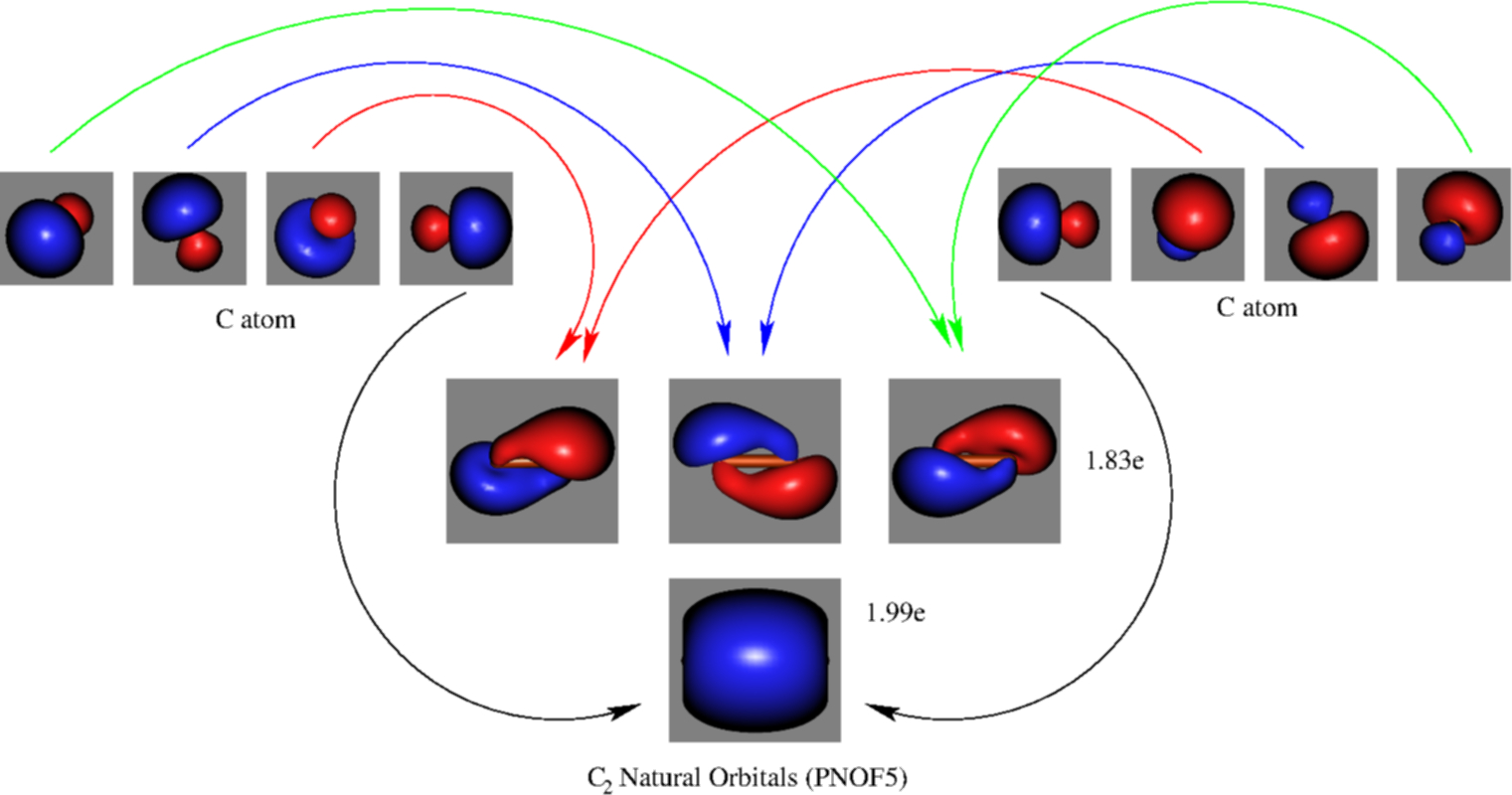


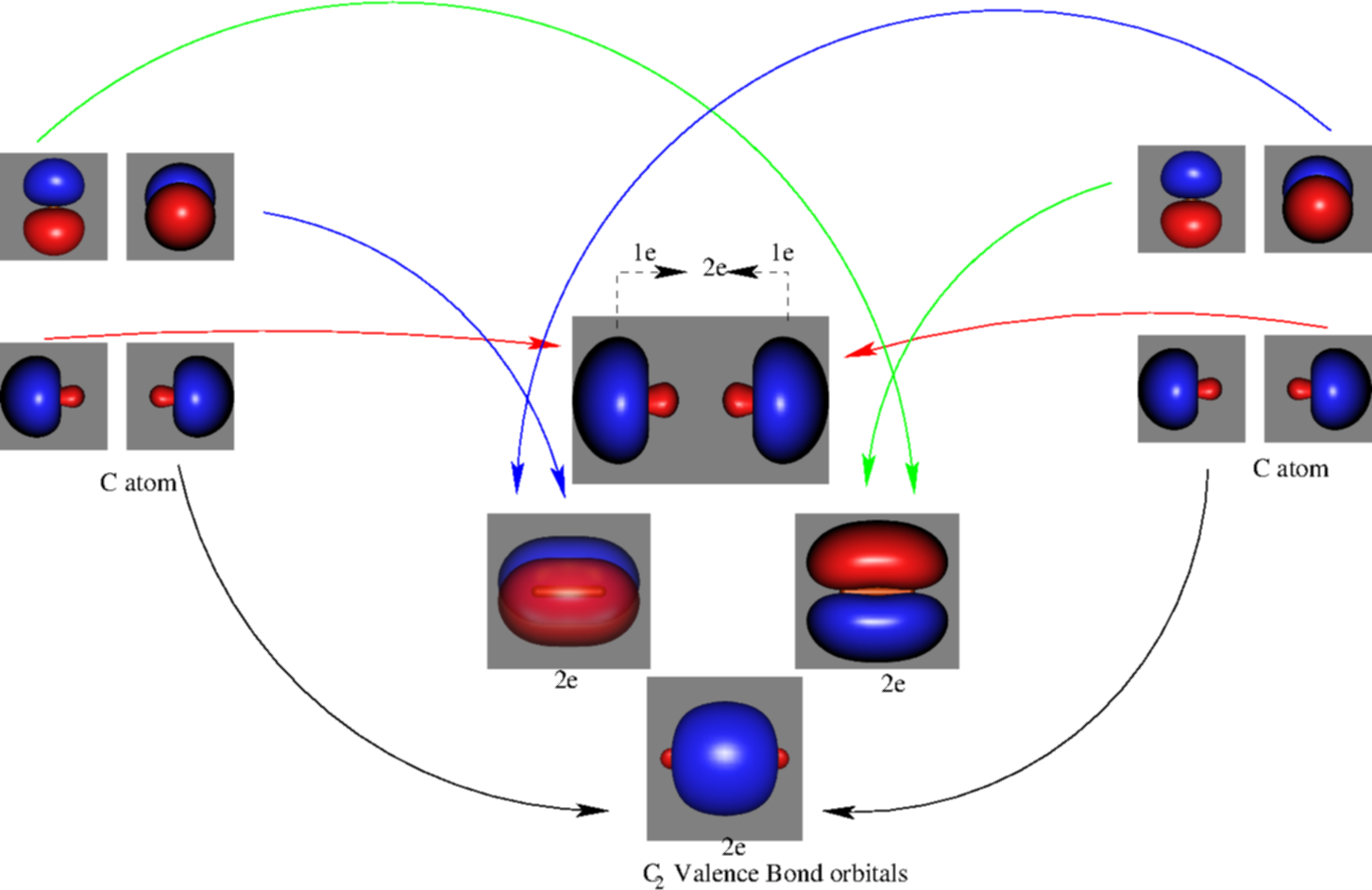










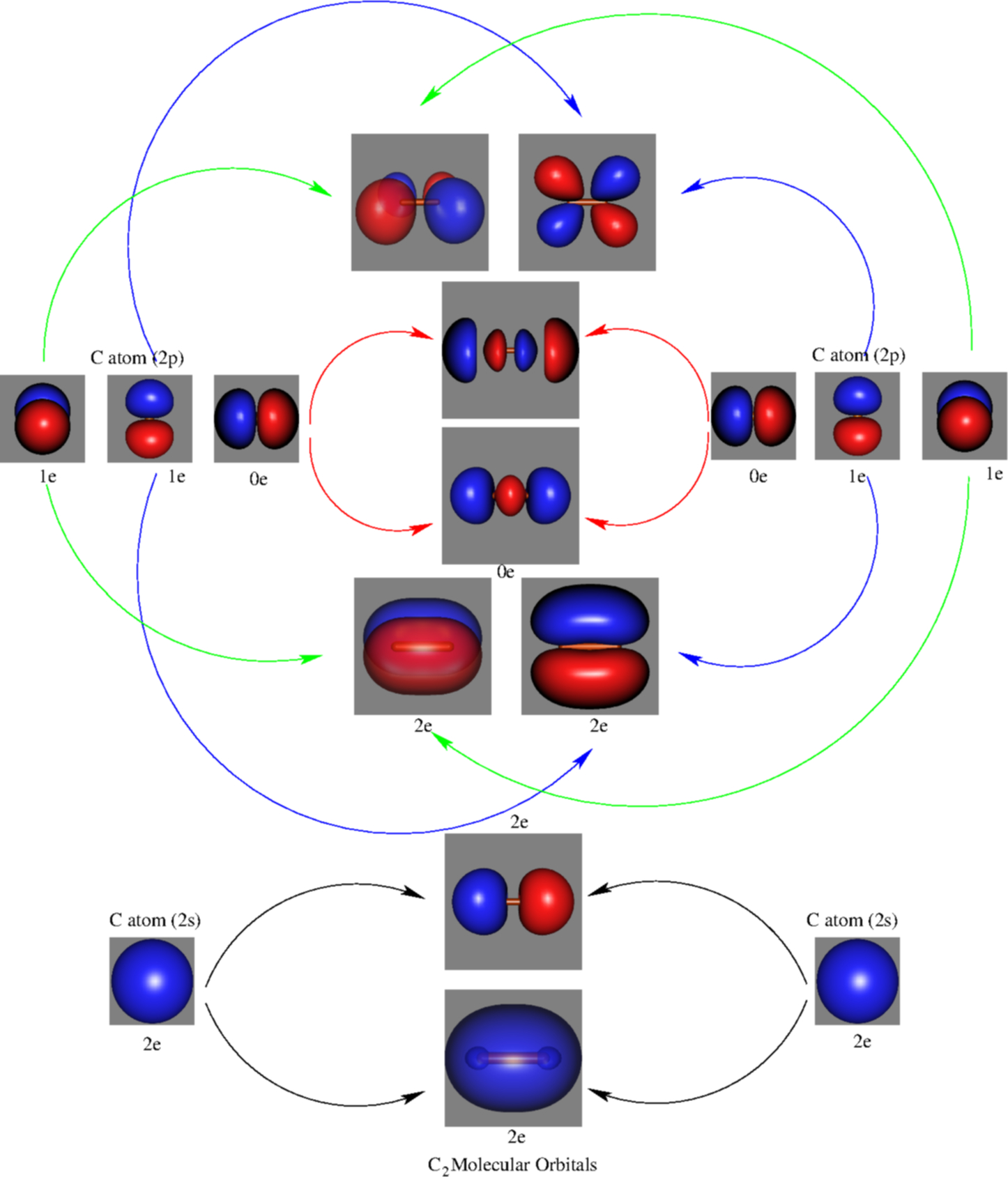
![Orbital descriptions of C2\documentclass[12pt]{minimal ...](https://www.researchgate.net/publication/337282871/figure/fig2/AS:958973922525208@1605648608866/Orbital-descriptions-of-C2documentclass12ptminimal-usepackageamsmath.png)
0 Response to "38 mo diagram for c2"
Post a Comment