39 orbital diagram for ni
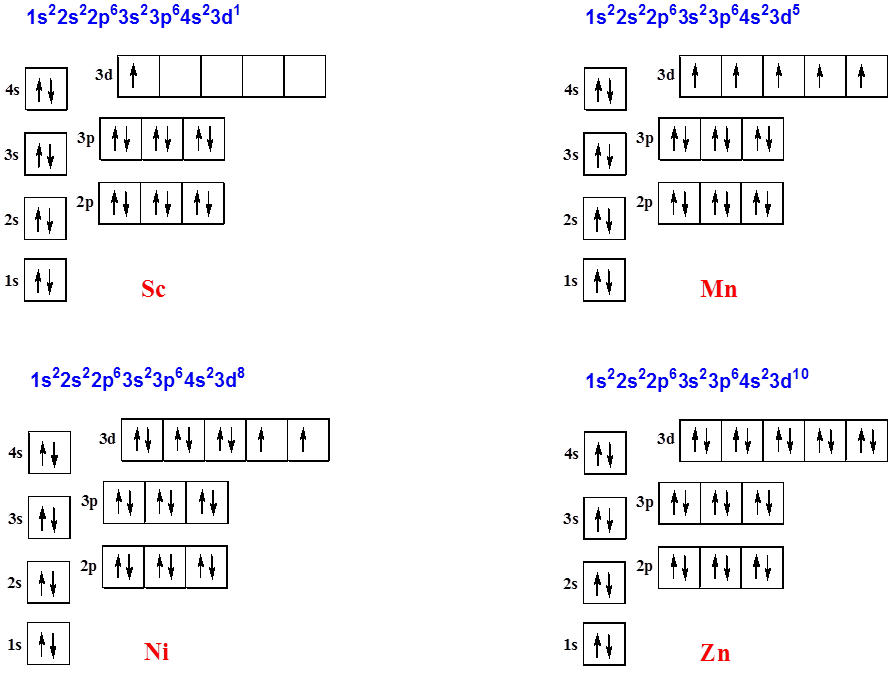
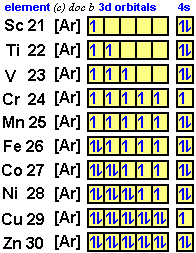
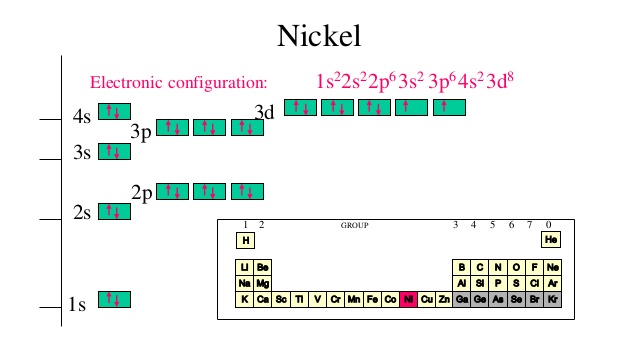
What is the orbital diagram for nickel? - Answers The orbital diagram for nickel is as follows: 1s2 2s2 2p6 3s2 3p6 4s2 3d8. In all of the cases, both up and down arrows are filled, with the exception of the 3d shell, where the last two are up ... techiescientist.com › c2h6-lewis-structureC2H6 Lewis Structure, Molecular Geometry, Hybridization ... 2 days ago · C2H6 Molecular Orbital (MO) Diagram. The molecular orbital theory, a quantum mechanical model, is used to draw the molecular orbital (MO) diagram of the ethane molecule. It is based on the linear combination of atomic orbitals, which lead to the formation of the molecular orbital. The molecular orbital diagram of ethane would be:
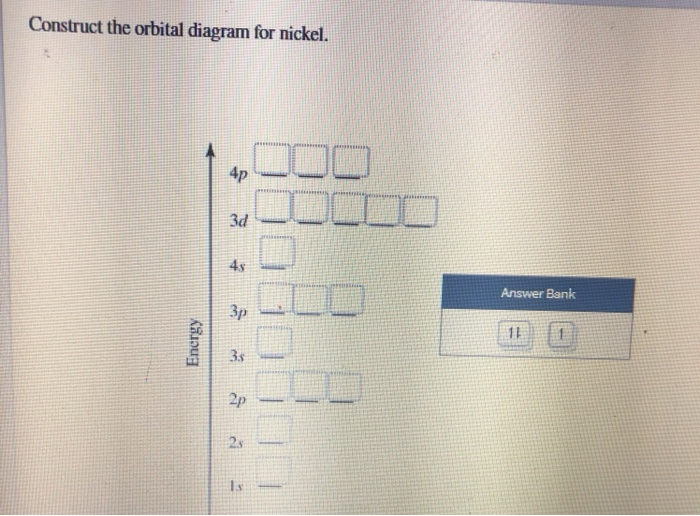
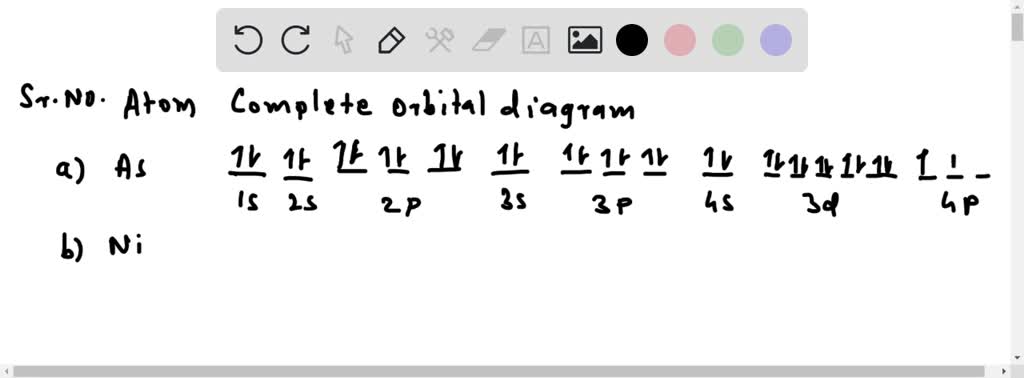
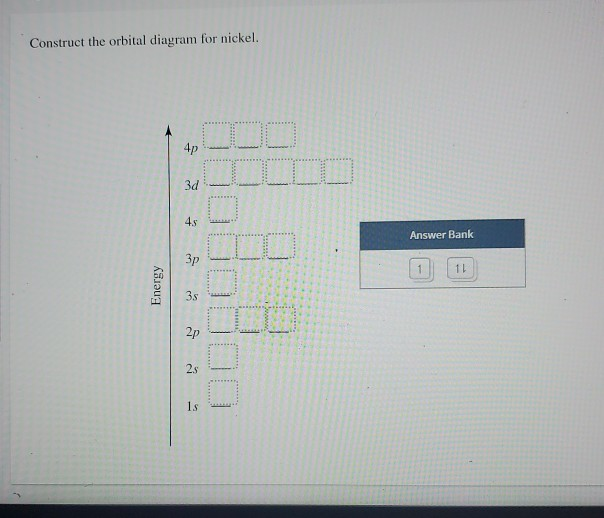
Construct the orbital diagram for Ni. - Clutch Prep Problem: Construct the orbital diagram for Ni. FREE Expert Solution Ni → atomic # 28 → 28 electrons. Ni will pass through 1s, 2s, 2p, 3s, 3p, 4s2, 3d. Following Aufbau principle (fill lowest energy first) and Hund's rule (half-filled first before totally filled) 82% (205 ratings)

Orbital diagram for ni
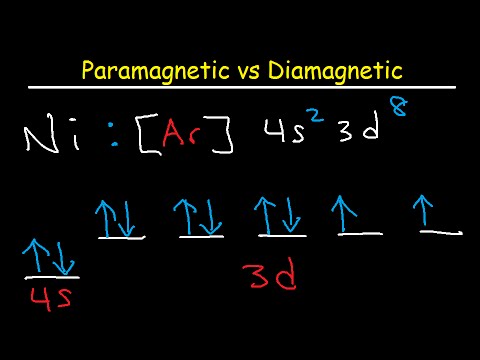
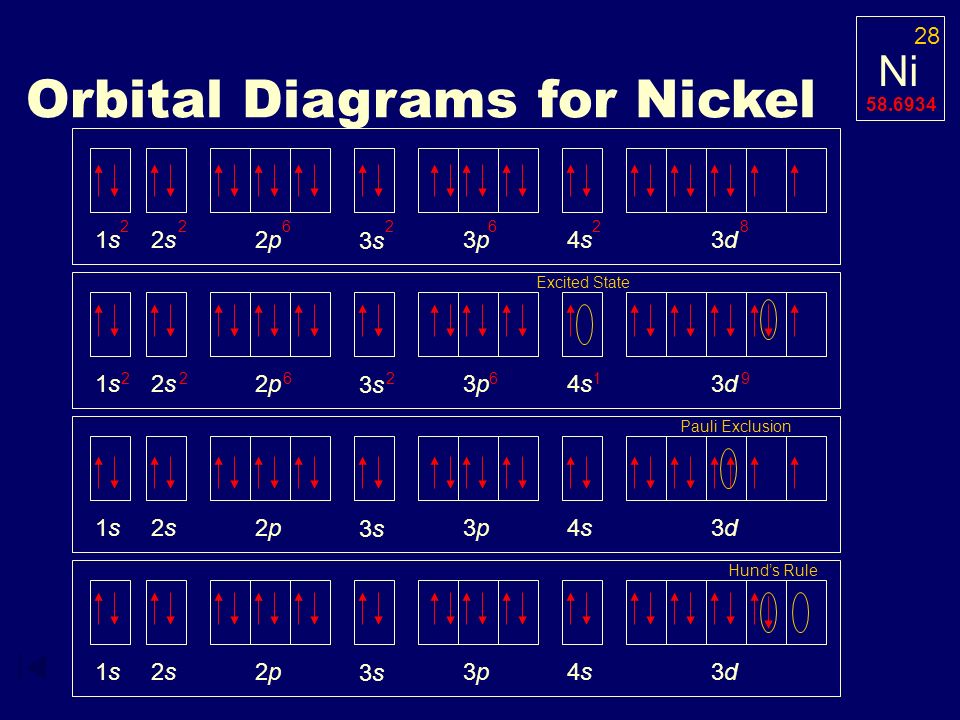
Orbital Diagram For Fe3+ Transition Fe3+ ions and draw the orbital box diagrams for both ions. Using this. There for 1s2 2s2 2p6 3s2 3p6 3d5 is the electronic configration for Fe3+. half of electrons (there must be one electron in each orbital, and d has 5 orbitals). That's for filling up orbitals for ground state atoms. QUESTION 24 Draw the orbital diagram for ... - Physical ... Question: QUESTION 24 Draw the orbital diagram for nickel (Ni). How many unpaired electrons does Ni have and is Ni paramagnetic or diamagn OneClass: For Ni2+, draw an orbital energy diagram and ... For Ni2+, draw an orbital energy diagram and place the valence electrons in the diagram that would indicate that it is in an excited state. Transition Metal Chemistry and Paper Chromatography would indicate that it is an excited state, Answer +20. Watch. 1. answer. 0. watching. 457. views.
Orbital diagram for ni. Orbital Diagrams - Concept - Chemistry Video by Brightstorm Orbital diagrams are a pictorial description of electrons in an atom. In order to figure out where electrons go in an atom we have to follow 3 main rules. The first one being the Auf Bau Principle, the Auf Bau Principle states that each electron occupies the lowest energy orbital available. Then we have to think okay with the sublevels, I mean ... Orbital Diagram Of Nickel - wireschema.com · The orbital diagram for nickel is as follows: 1s2 2s2 2p6 3s2 3p64s2 3d8. In all of the cases, both up and down arrows are filled,with the exception of the 3d shell. Nickel is atomic number 28; therefore, it has 28 electrons in its . opg.optica.org › oe › abstractChiro-optical fields with asymmetric orbital angular momentum ... Feb 18, 2022 · In this paper, we proposed a flexible method for generating asymmetric chiro-optical fields. Different from most of the chiro-optical fields superimposed by vortex beams which are rotationally symmetric, the asymmetric chiro-optical field has a locally controllable orbital angular momentum (OAM) and polarization state. By using a helix phase plate (HPP) calculated based on coordinates ... chemed.chem.purdue.edu › genchem › topicreviewQuantum Numbers and Electron Configurations The fourth orbital in this subshell lies along the X and Y axes and is called the 3d x 2-y 2 orbital. Most of the space occupied by the fifth orbital lies along the Z axis and this orbital is called the 3d z 2 orbital. The number of orbitals in a shell is the square of the principal quantum number: 1 2 = 1, 2 2 = 4, 3 2 = 9.
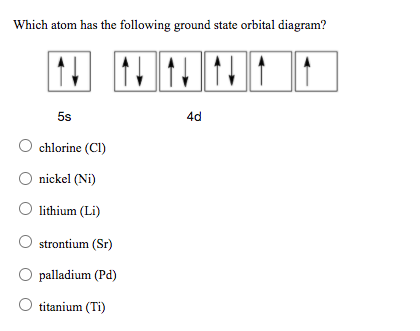
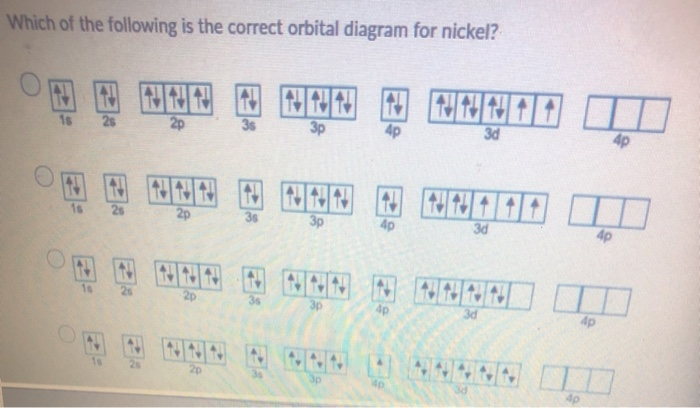
Solved Choose the valence orbital diagram that represents ... Chemistry questions and answers. Choose the valence orbital diagram that represents the ground state of Ni. 43 3d 11 34 11 13 25 2p 11 45 11|11|1|11 3d 45. What is the orbital diagram for nickel? | Study.com What is the orbital diagram for nickel? Atomic Orbital Diagrams: Atomic orbital diagrams are also known as electron-in-a-box diagrams. These are simplified diagrams of how electrons are arranged ... How to Do Orbital Diagrams - Sciencing Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics. How to Draw Orbital Diagrams - YouTube Orbital diagrams are a visual way to show where the electrons are located within an atom. Orbital diagrams must follow 3 rules: The Aufbau principle, the Pau...
How would we know that "Ni"("CO")_4 prefers tetrahedral ... Ni(CO)4 has nickel in its 0 oxidation state, with electron configuration [Ar]3d84s2. So, we call it a d10 complex in the ligand field. Here is its MO diagram (it is tetrahedral ): Here, the 2e and 9t2 orbitals are what we pick out as the d -orbital splitting diagram with tetrahedral splitting energy Δt. The rest comes from ligand field theory. Complete the atomic orbital diagram for Nickel (Ni) and ... Complete the atomic orbital diagram for Nickel (Ni) and answer the following questions (assume a ground state electron configuration): (8 points) 1s 2s 2p 3s 3p 4s 3d . a) Write the complete ground state electron configuration for Nickel in the space below: b) Write the ground state electron configuration of Nickel in Noble Gas Notation below: PDF Electron Configurations and Orbital Diagrams key Write the electron configuration (full, and in core notation) for the following ions: 1.-1Br +3 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 [Kr], [Ar] 3d 10 4s 2 4p 6 2. Sr +2 8. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4s [Kr], [Ar] 3d 10 4s 2 4p 6 3. +2Se-2 9. 1s 2 2s2 2p6 3s 2 3p 6 3d 10 4s 2 4p 6 [Kr], [Ar] 3d 10 4s 2 4p 6 4. periodictableguide.com › orbital-diagram-of-allOrbital Diagram of All Elements (Diagrams given Inside) Apr 10, 2021 · Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13 ...
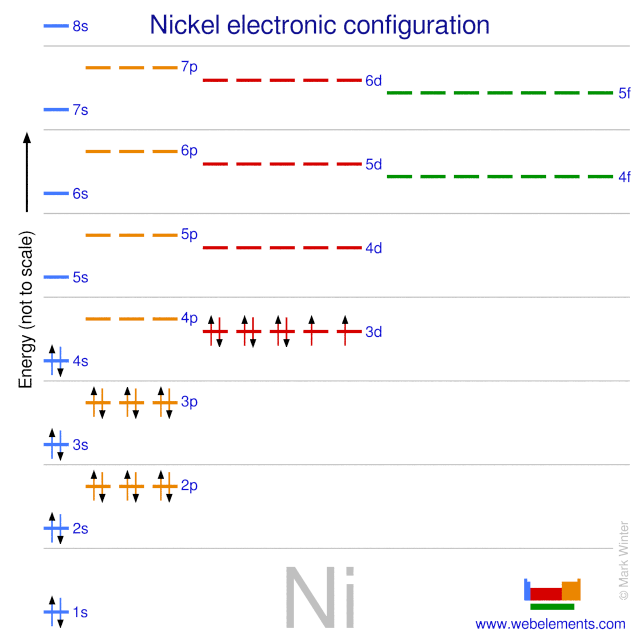
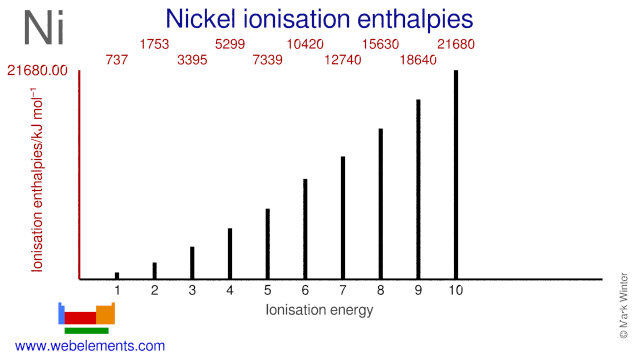
Electron configuration for Nickel (element 28). Orbital ... Ni (Nickel) is an element with position number 28 in the periodic table. Located in the IV period. Melting point: 1453 ℃. Density: 8.91 g/cm 3 . Electronic configuration of the Nickel atom in ascending order of orbital energies: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 8. Electronic configuration of the Nickel atom in ascending order of the levels:
en.wikipedia.org › wiki › Tanabe–Sugano_diagramTanabe–Sugano diagram - Wikipedia This could also be described as a positive "hole" that moves from the e g to the t 2g orbital set. The sign of Dq is opposite that for d 1, with a 2 E g ground state and a 2 T 2g excited state. Like the d 1 case, d 9 octahedral complexes do not require the Tanabe–Sugano diagram to predict their absorption spectra.
Beryllium Orbital Diagram - schematron.org Beryllium Orbital Diagram. A quiz solution for Inorganic Chemistry in which students were prompted to draw the molecular orbital diagram for beryllium hydride. Beryllium is the fourth element with a total of 4 electrons. In writing the electron configuration for beryllium the first two electrons will go in the 1s orbital.
PDF Orbital Diagrams, Noble Gas Configuration, Lewis Dot Diagrams Orbital Filling Diagrams •Each box represents an orbital which can hold a max of 2 e- •Aufbau principal -each electron occupies the lowest energy orbital available; German for "build up" •Electrons are notated with an arrow -Up arrow goes first then, down arrow -Arrows represent the opposing spin of electrons 5.2 Quantum Theory & The Atom
PDF MO Diagrams for More Complex Molecules • MO diagrams can be built from group orbitals and central atom orbitals by considering orbital symmetries and energies. • The symmetry of group orbitals is determined by reducing a reducible representation of the orbitals in question. This approach is used only when the group orbitals are not obvious by inspection.
Niobium(Nb) electron configuration and orbital diagram Niobium (Nb) orbital diagram 1s is the closest and lowest energy orbital to the nucleus. Therefore, the electron will first enter the 1s orbital. According to Hund's principle, the first electron will enter in the clockwise direction and the next electron will enter the 1s orbital in the anti-clockwise direction.
Why is "Ni"("CN")_4^(2-) diamagnetic but "NiCl"_4^(2 ... Their blank d -splitting diagrams within the realm of crystal field theory are: [Ni(CN)4]2−: The d orbitals fill with 8 electrons, then, with a low spin configuration. You can see that an even number of d orbitals will get filled ( dyz,dxz,dz2,dxy) with an even number of 3d electrons. This gives rise to a diamagnetic configuration, as expected.
What is the orbital diagram for nickel? - Quora Nickel is in the 4th energy level, d block, 7th column, this means that the electron configuration will end 3d8 with the d orbital being one level lower than the energy level it is on. Ni=1s22s22p63s23p64s23d8 Ni= [Ar]4s23d8 12.9K views View upvotes Related Answer Mike Jones , MAEd Chemistry & Physics, Western Carolina University (1974)
Orbital Diagrams and Electron Configuration - Basic ... This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n...
en.wikipedia.org › wiki › Linear_combination_ofLinear combination of atomic orbitals - Wikipedia A linear combination of atomic orbitals or LCAO is a quantum superposition of atomic orbitals and a technique for calculating molecular orbitals in quantum chemistry. In quantum mechanics, electron configurations of atoms are described as wavefunctions.
Construct The Orbital Diagram For Ni Construct the orbital diagram for Ni. Be able to construct molecular orbital diagrams for homonuclear diatomic, heteronuclear diatomic, homonuclear triatomic, and heteronuclear triatomic molecules. Understand and be able to articulate how molecular orbitals form - conceptually, visually, graphically, and (semi)mathematically.
schmeling.ac.rwth-aachen.de › cohp › indexCOHP - Crystal Orbital Hamilton Population • Theory For Cr (a typical antiferromagnet), the Fermi level lies in the COHP curve between the bonding and antibonding regions, whereas for the metals from Mn (which we describe in a bcc structure for simplicity) to Ni, the Fermi level lies in the clearly antibonding region; Fe, Co, and Ni are all ferromagnetic.
Nickel(Ni) electron configuration and orbital diagram To write the orbital diagram of nickel(Ni), you have to do the electron configuration of nickel. Which has been discussed in detail above. Nickel(Ni) orbital diagram. 1s is the closest and lowest energy orbital to the nucleus. Therefore, the electron will first enter the 1s orbital. According to Hund's principle, the first electron will enter ...
Construct The Orbital Diagram For Ni Nickel (Ni) has an atomic mass of Find out about its chemical and physical properties, states, energy, electrons, oxidation and more. Orbital Diagram. 1s (Ni,Fe)9S8] ore. The metal is produced by heating the ore in a blast furnace which replaces the sulfur with oxygen. The oxides are then treated with an acid that reacts with the. 1.
The Orbital Diagram for Ni Stories The Orbital Diagram for Ni Stories The methods to acquire an atomic orbital with the correct character for the bonding is known as hybridization. Frequently, the bonding atomic orbitals have a character of numerous possible kinds of orbitals.
Construct the orbital diagram for Ni. - Clutch Prep We're being asked to construct the orbital diagram for Ni. For that, we first need to determine the electron configuration of Ni. Recall that for a neutral element, Atomic number = # of protons = # of electrons. The atomic number of Ni is 28 and since it's a neutral element, this means Ni has 28 electrons. 95% (479 ratings)
OneClass: For Ni2+, draw an orbital energy diagram and ... For Ni2+, draw an orbital energy diagram and place the valence electrons in the diagram that would indicate that it is in an excited state. Transition Metal Chemistry and Paper Chromatography would indicate that it is an excited state, Answer +20. Watch. 1. answer. 0. watching. 457. views.
QUESTION 24 Draw the orbital diagram for ... - Physical ... Question: QUESTION 24 Draw the orbital diagram for nickel (Ni). How many unpaired electrons does Ni have and is Ni paramagnetic or diamagn
Orbital Diagram For Fe3+ Transition Fe3+ ions and draw the orbital box diagrams for both ions. Using this. There for 1s2 2s2 2p6 3s2 3p6 3d5 is the electronic configration for Fe3+. half of electrons (there must be one electron in each orbital, and d has 5 orbitals). That's for filling up orbitals for ground state atoms.
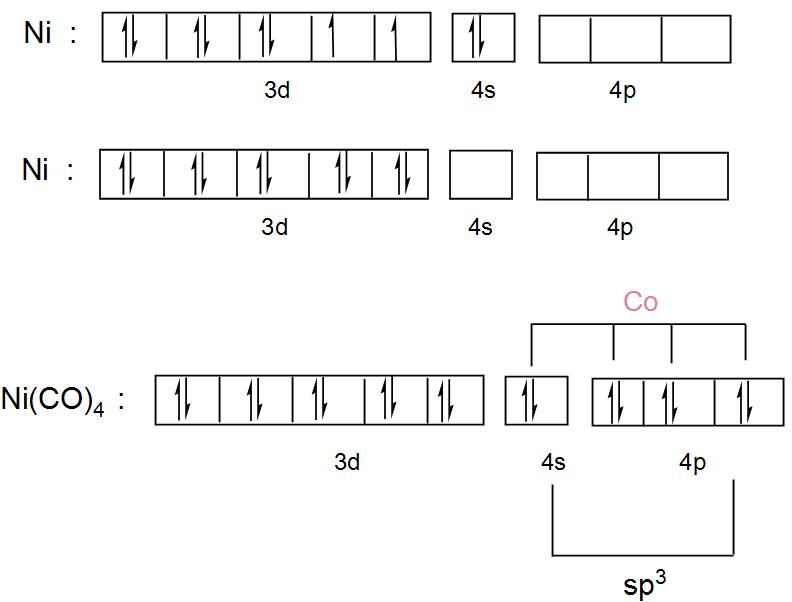















![Hybridization-Ni(CO)4 | [Ni(CN)4]2-| [Ni(Cl)4]2- | Structure ...](https://www.adichemistry.com/jee/qb/coordination-chemistry/1/q1-4.png)


0 Response to "39 orbital diagram for ni"
Post a Comment