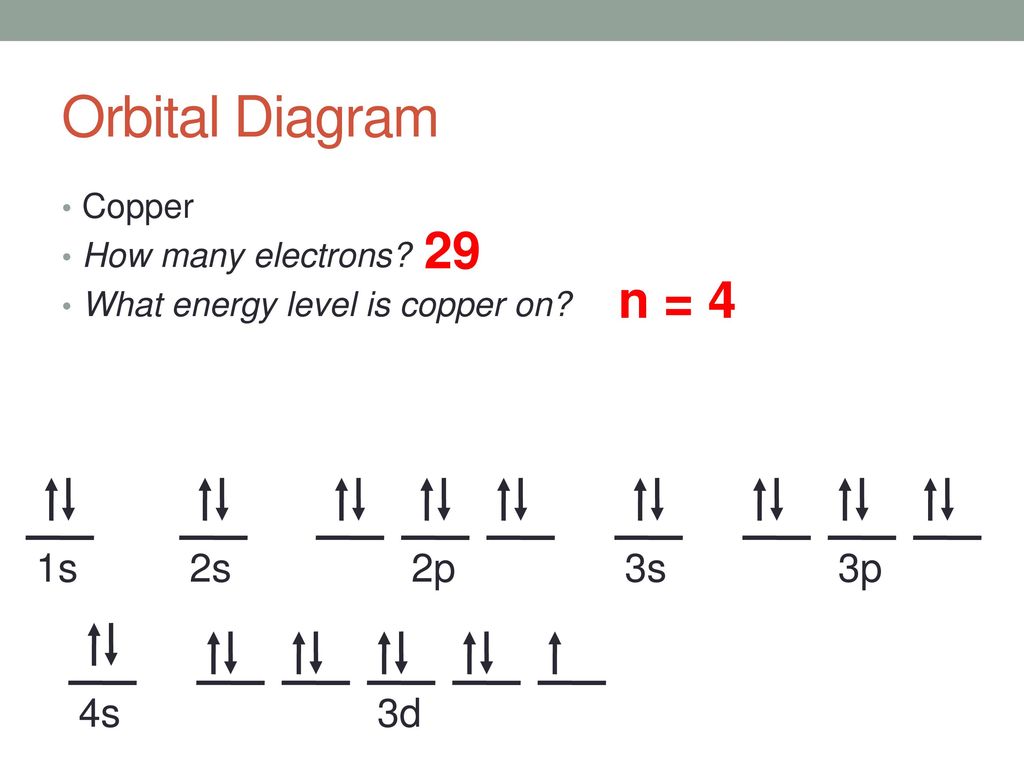
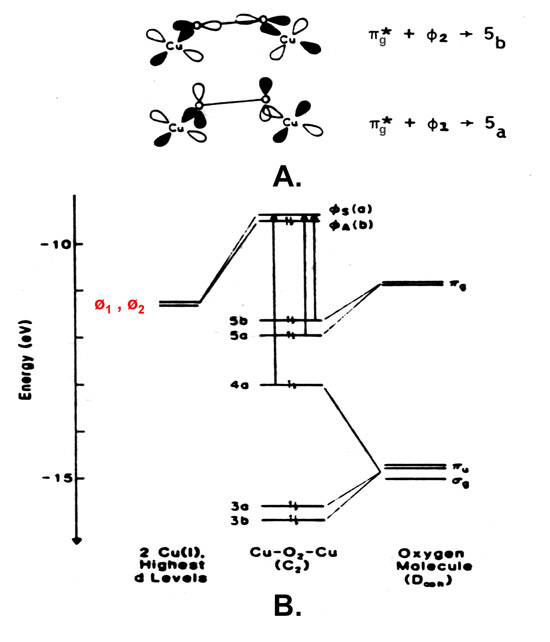
40 orbital diagram for copper
Chlorine(Cl) electron configuration and orbital diagram Orbital diagram for chlorine(Cl) Chlorine(Cl) excited state electron configuration. Atoms can jump from one orbital to another orbital by excited state. This is called quantum jump. Ground state electron configuration of chlorine is 1s 2 2s 2 2p 6 3s 2 3p 5. The valency of the element is determined by electron configuration in the excited state. 41 bohr diagram for copper - Wiring Diagram Trend When a manganese atom is excited, then the manganese atom absorbs energy. As a result, an electron in the 4s orbital jumps to the 4p x sub-orbital. The p-orbital has three sub-orbitals. The sub-orbitals are p x, p y, and p z. Bohr diagram for copper
Copper (Cu) - ChemicalAid Copper (Cu) has an atomic mass of 29. Find out about its chemical and physical ... Orbital Diagram. Cu - Copper - Orbital Diagram - Electron Configuration ...
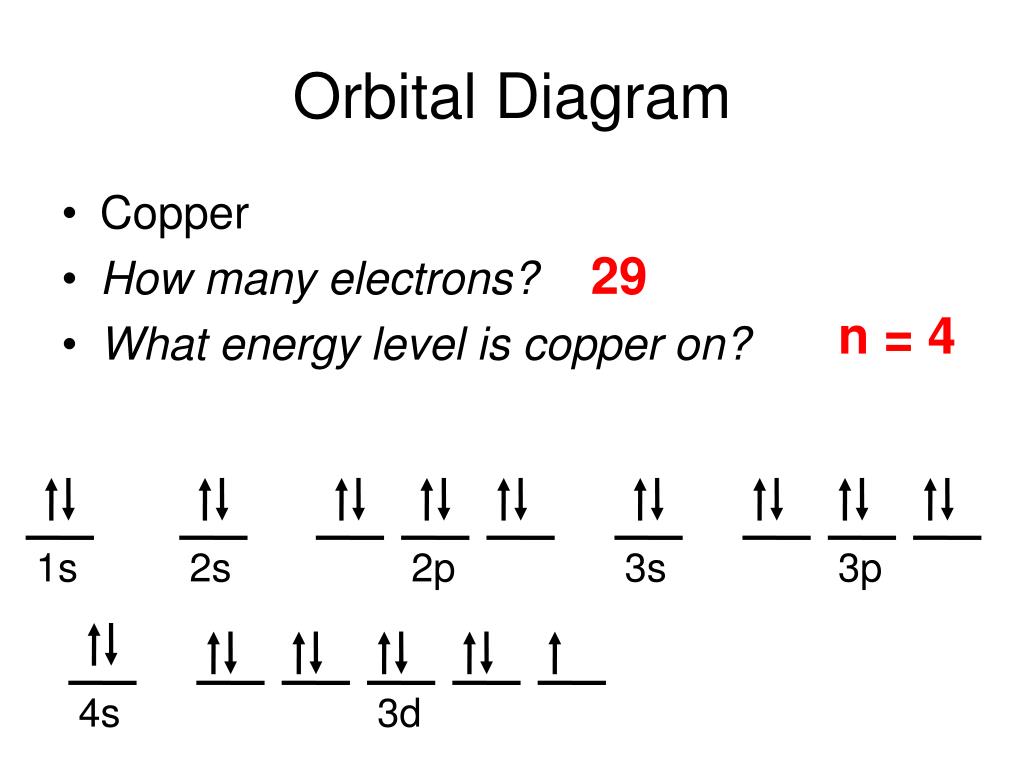
Orbital diagram for copper
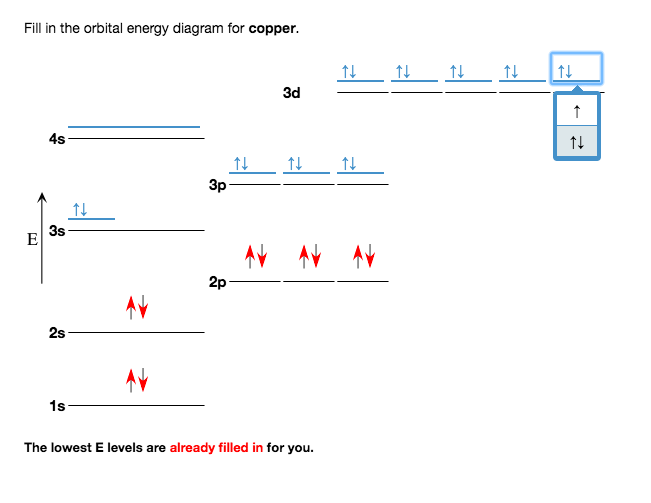
Fill in the orbital energy diagram for cop... | Clutch Prep We're being asked to fill the orbital energy diagram for copper. Before we can do that, we have to first identify the number of electrons. • In a neutral atom: Atomic number = # of protons = # of electrons. Cu: atomic number = 29 → 29 protons & 29 electrons. • Distribute electrons in the atomic orbitals: 97% (358 ratings) Electron configuration for Copper (element 29). Orbital ... Cu (Copper) is an element with position number 29 in the periodic table. Located in the IV period. Melting point: 1083.5 ℃. Density: 8.92 g/cm 3 . The order of filling the orbitals with electrons in the Cu atom is an exception to the rule. Expected electronic configuration. 1s2 2s2 2p6 3s2 3p6 4s2 3d9. But in reality, one electron moves from ... What is the orbital diagram for copper? - Answers Definition of orbital diagram? An orbital diagram is used to show how the orbitals of a subshell areoccupied by electrons. The two spin projections are given by arrowspointing up (ms =+1/2) and...
Orbital diagram for copper. Tellurium(Te) electron configuration and orbital diagram Tellurium(Te) is the 52nd element in the periodic table and its symbol is 'Te'. This article gives an idea about the electron configuration of tellurium and orbital diagram, period and groups, valency and valence electrons of tellurium, bond formation, compound formation, application of different principles. Electron Configuration for Cu, Cu+, and Cu2+ (Copper and ... To write the configuration for the Copper ions, first we need to write the electron configuration for just Copper (Cu). We first need to find the number of ... What is the complete electron configuration of copper(I ... A copper (I) ion is basically a copper ion with an oxidation state of +1, i.e. it will become Cu+. Copper has an electron configuration of [Ar]3d104s1. 1s22s22p63s23p63d104s1. When it loses that 1 electron, it no longer needs the 4s orbital, and therefore its electron configuration becomes. 1s22s22p63s23p63d10. What is the electron configuration of copper? | Socratic Copper is in the ninth column of the transition metals in the d block of the fourth energy level of the periodic table.
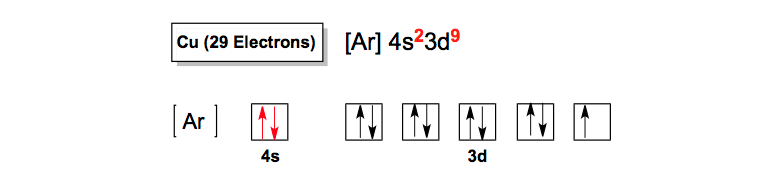
Draw and explain the orbital diagram for copper (Z = 29 ... Answer to: Draw and explain the orbital diagram for copper (Z = 29). By signing up, you'll get thousands of step-by-step solutions to your homework... Copper(Cu) electron configuration and orbital diagram Atomic Orbital Diagram for Copper (Cu) Copper ion(Cu +,Cu 2+) electron configuration. Ground state electron configuration of copper(Cu) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 1. The electron configuration shows that the last shell of copper has an electron and the d-orbital has a total of ten electrons. In this case, the valence electrons of copper are one. There are two types of copper ions. Copper Electron Configuration (Cu) with Orbital Diagram Jan 26, 2021 — If the general pattern of filling electron orbitals is followed, then copper's electron configuration is 1s2 2s2 2p6 3s23p6 4s2 3d9. The only ... Niobium(Nb) electron configuration and orbital diagram That is, niobium is a cation element. Nb - 5e - → Nb 5+. The electron configuration of niobium ion (Nb 5+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6. The electron configuration of a niobium ion shows that the niobium ion (Nb 5+) has four shells and the last shell has eight electrons.
9.9.9B: Orbital Energies - Chemistry LibreTexts For example, copper has an electron configuration of [Ar]4s 1 d 10. This configuration, which is at odds with the simple mnemonic, would be predicted successfully by the orbital ordering for copper given in an orbital energy diagram. Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagram of Copper (Cu) 30: Orbital diagram of Zinc (Zn) 31: Orbital diagram of Gallium (Ga) 32: Orbital diagram of Germanium (Ge) 33: Orbital diagram of Arsenic (As) 34: Orbital diagram of Selenium (Se) 35: Orbital diagram of Bromine (Br) 36: Orbital diagram of Krypton (Kr) 37: Orbital diagram of Rubidium (Rb) 38: What is the orbital diagram for copper? - Answers The electron configuration of copper is 1s22s22p63s23p63d104s1. The Order of Filling 3d and 4s Orbitals - Chemistry LibreTexts The diagram (not to scale) summarizes the energies of the orbitals up to the 4p level. Figure 1: Electronic energies orbitals. The oddity is the position of the 3d orbitals, which are shown at a slightly higher level than the 4s. This means that the 4s orbital which will fill first, followed by all the 3d orbitals and then the 4p orbitals.
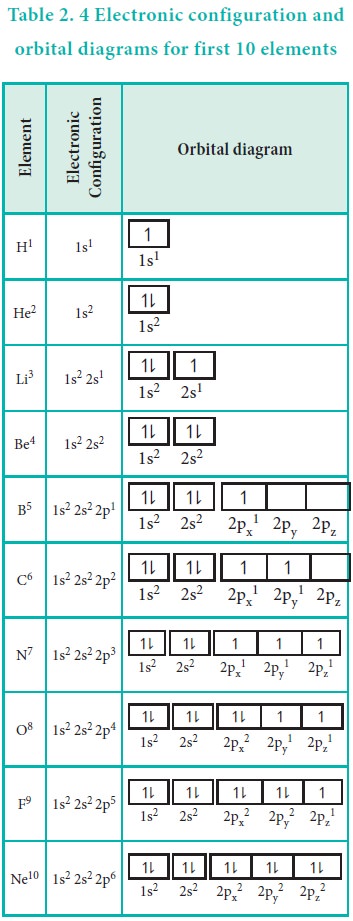
Electron Configurations, Orbital Box Notation (M7Q7 ... The 1s orbital at the bottom of the diagram is the orbital with electrons of lowest energy. The energy increases as we move up to the 2 s and then 2 p , 3 s , and 3 p orbitals, showing that the increasing n value has more influence on energy than the increasing l value for small atoms.
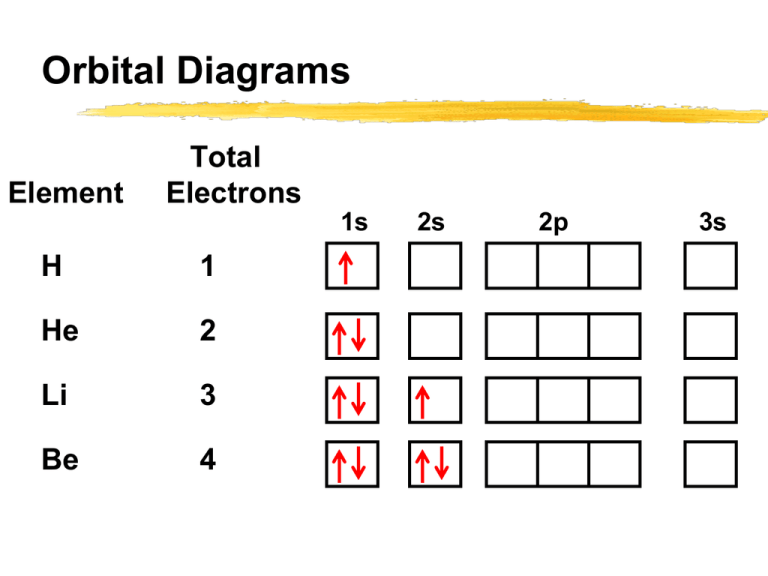
PDF Orbital Diagrams, Noble Gas Configuration, Lewis Dot Diagrams Orbital Filling Diagrams •Each box represents an orbital which can hold a max of 2 e- •Aufbau principal -each electron occupies the lowest energy orbital available; German for "build up" •Electrons are notated with an arrow -Up arrow goes first then, down arrow -Arrows represent the opposing spin of electrons 5.2 Quantum Theory & The Atom
Electron Configuration for Copper (Cu, Cu+, Cu2+) Since 1s can only hold two electrons the next 2 electrons for Copper go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next two electrons in the 3s. Since the 3s if now full we'll move to the 3p where we'll place the next six electrons.
Solved a) Write the full electron configuration for Copper ... Chemistry questions and answers. a) Write the full electron configuration for Copper. b) Write the full orbital diagram for Copper. c) Write the abbreviated electron configuration for Copper. d) How many valence electrons does Copper have? Question: a) Write the full electron configuration for Copper. b) Write the full orbital diagram for Copper.
PDF Electron Configurations, Orbital Notation and Quantum Numbers Start by drawing its orbital notation for the outermost, valence electrons. [Ne] ↑↓ ↑↓ ↑ ↑ 3s 3p Sulfur is a nonmetal and tends to gain electrons, creating the -2 charge. Gaining two electrons gives it an octet of 3s23p6. • Copper has two common oxidation states, +1 and +2.
What is the orbital diagram for copper? - Answers Definition of orbital diagram? An orbital diagram is used to show how the orbitals of a subshell areoccupied by electrons. The two spin projections are given by arrowspointing up (ms =+1/2) and...
Electron configuration for Copper (element 29). Orbital ... Cu (Copper) is an element with position number 29 in the periodic table. Located in the IV period. Melting point: 1083.5 ℃. Density: 8.92 g/cm 3 . The order of filling the orbitals with electrons in the Cu atom is an exception to the rule. Expected electronic configuration. 1s2 2s2 2p6 3s2 3p6 4s2 3d9. But in reality, one electron moves from ...
Fill in the orbital energy diagram for cop... | Clutch Prep We're being asked to fill the orbital energy diagram for copper. Before we can do that, we have to first identify the number of electrons. • In a neutral atom: Atomic number = # of protons = # of electrons. Cu: atomic number = 29 → 29 protons & 29 electrons. • Distribute electrons in the atomic orbitals: 97% (358 ratings)











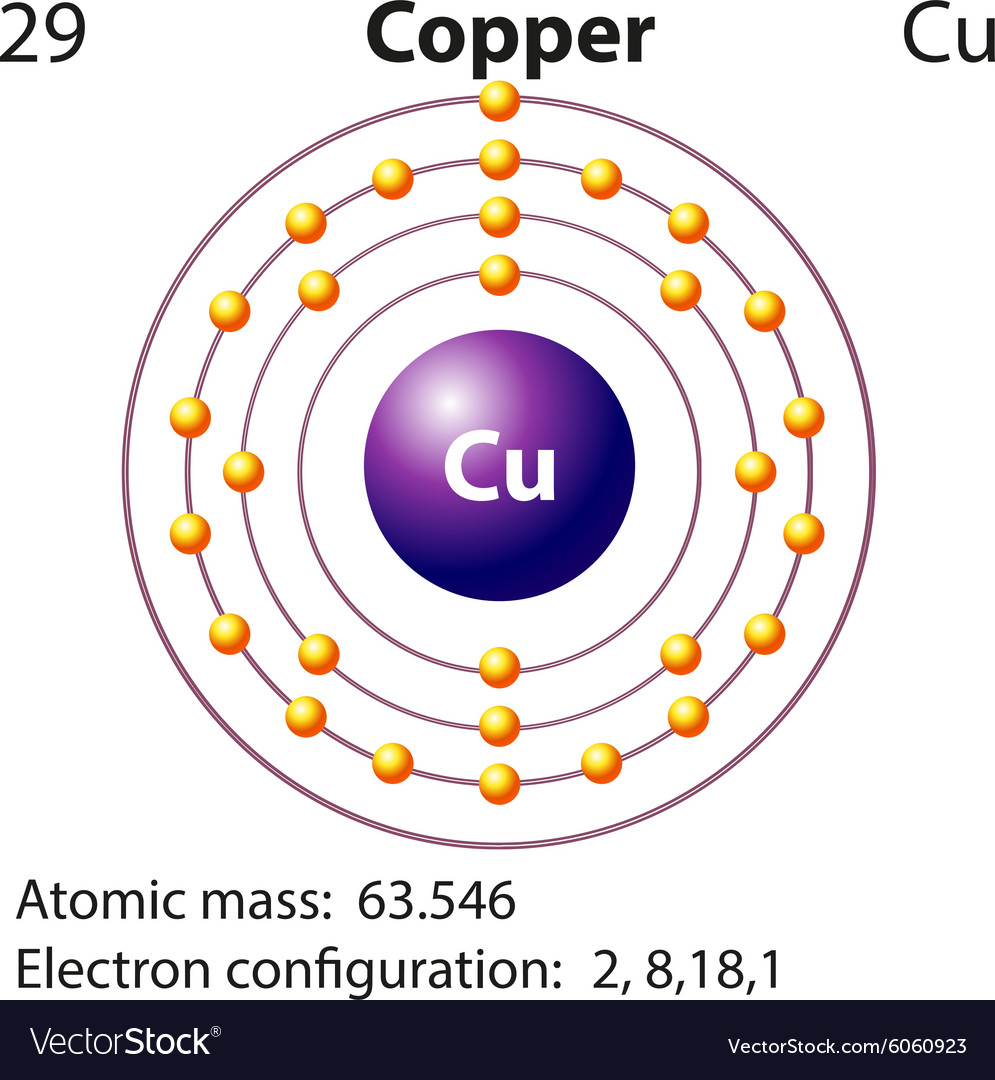












/800px-Orbital_representation_diagram.svg-589bd6285f9b58819cfd8460.png)

0 Response to "40 orbital diagram for copper"
Post a Comment