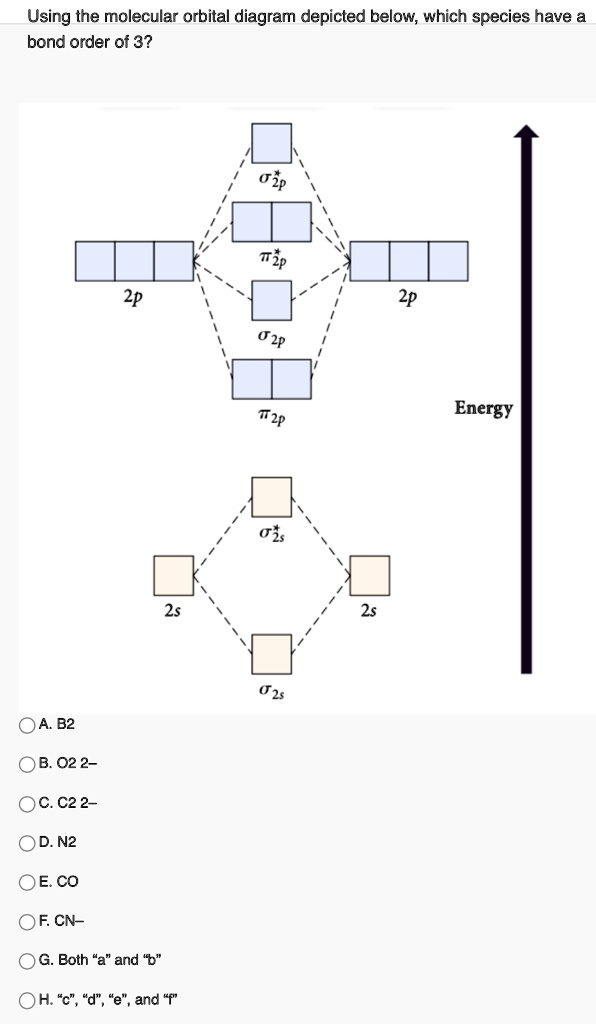
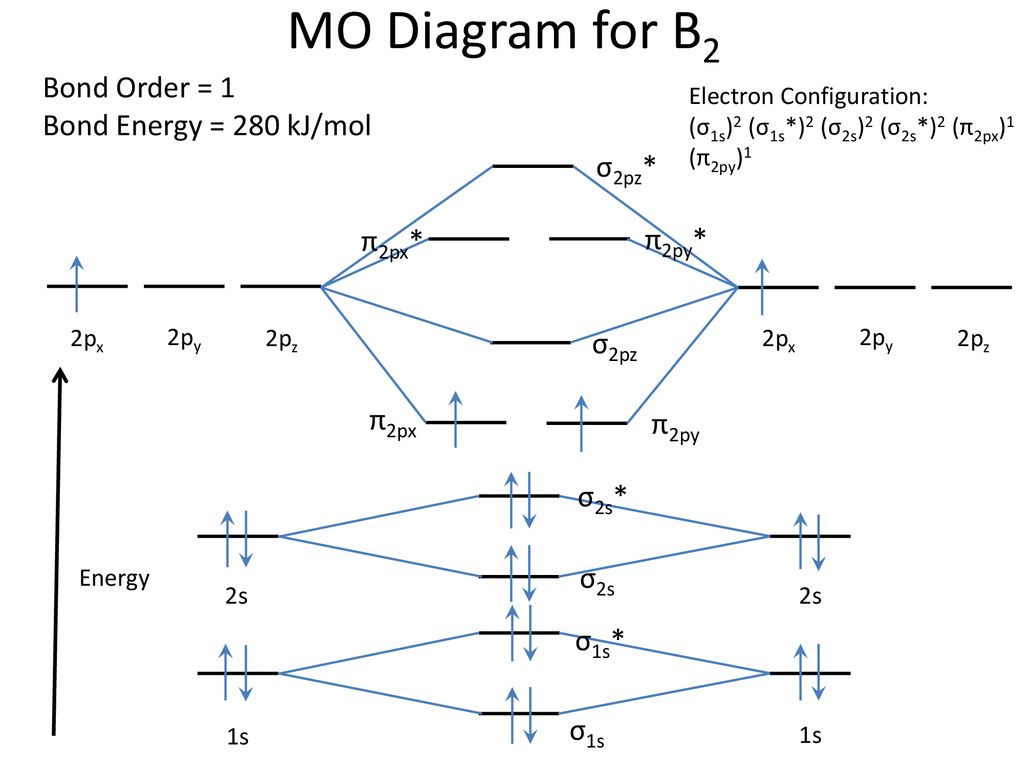
41 molecular orbital diagram for b2
Molecular orbital diagram of B2 1 See answer manfoosah2002 is waiting for your help. Add your answer and earn points. priyesharma02 priyesharma02 Molecular orbita diagram of B2 New questions in Chemistry. ejj-bgir-qdr hajqjqbwvgwhw short note on all the types of Quantum numbers. Which of the following is paramagnetic? (use the molecular orbital diagram) a) Li 2. b) Be 2. c) B 2. d) C 2. e) N 2. Learn this topic by watching MO Theory: Homonuclear Diatomic Molecules Concept Videos.
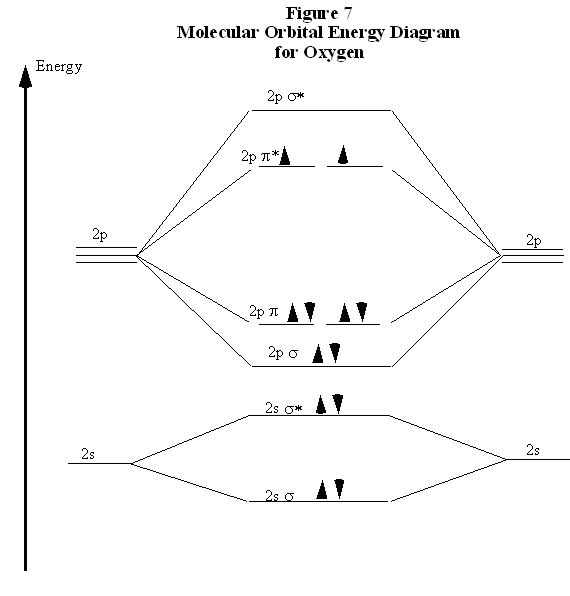
Molecular Orbital Diagrams, Bond Order, and Number of Unpaired Electrons Draw the molecular orbital diagram for the oxygen molecule, O 2. From this diagram, calculate the bond order for O 2. How does this diagram account for the paramagnetism of O 2? Solution. We draw a molecular orbital energy diagram similar to that shown in Figure 11.
Molecular orbital diagram for b2
After reading the theory part draw the MO diagrams for the ... B2. C2. N2. O2. Ne2. F2 electron configuration. N. OF. OCCUPIED. MO. N. of bonding electrons1. Firstly the molecular orbital diagram is drawn then bond order is calculated using the bonding and antibonding electrons. After that, check for its magnetic ... From the periodic table as we have already discussed the Molecular orbital diagrams of diatomic molecules of 1st two periods starting from Hydrogen to Neon. ...
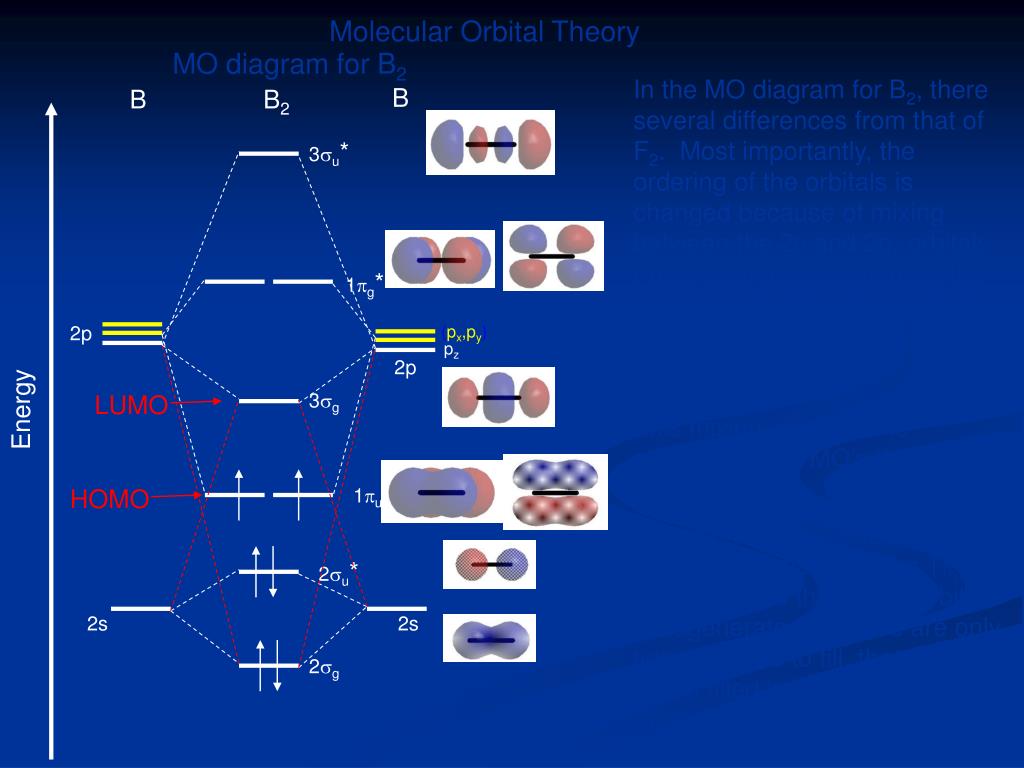
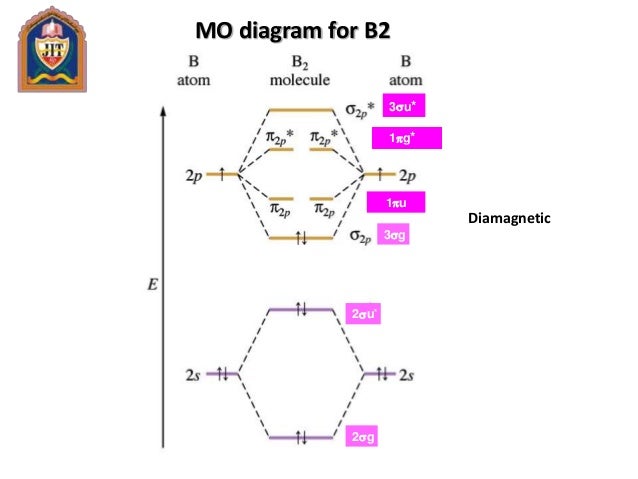
Molecular orbital diagram for b2. Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ... Nov 09, 2014 · Re: M.O. Diagram for B2 Post by Chem_Mod » Tue Nov 11, 2014 11:21 pm As discussed in class the MO diagram for B 2 shows that it has two unpaired electrons (which makes it paramagnetic) and these electrons are in bonding molecular orbitals resulting in the equivalent bond strength of one bond. In MO theory, bond order is half the difference between the numbers of electrons in the bonding and antibonding molecular orbitals. In NO there are 10 electrons ... The unhybridized 2p1 orbital lies perpendicular to the three hybridised orbitals. Representation of sp 2 hybridization sp 2 hybridization is also known as trigonal hybridisation. Each sp 1 hybrid orbital has s-character and The molecular orbital structure of ethylene: In ethene molecule, each carbon atom undergoes sp 2 hybridisation.
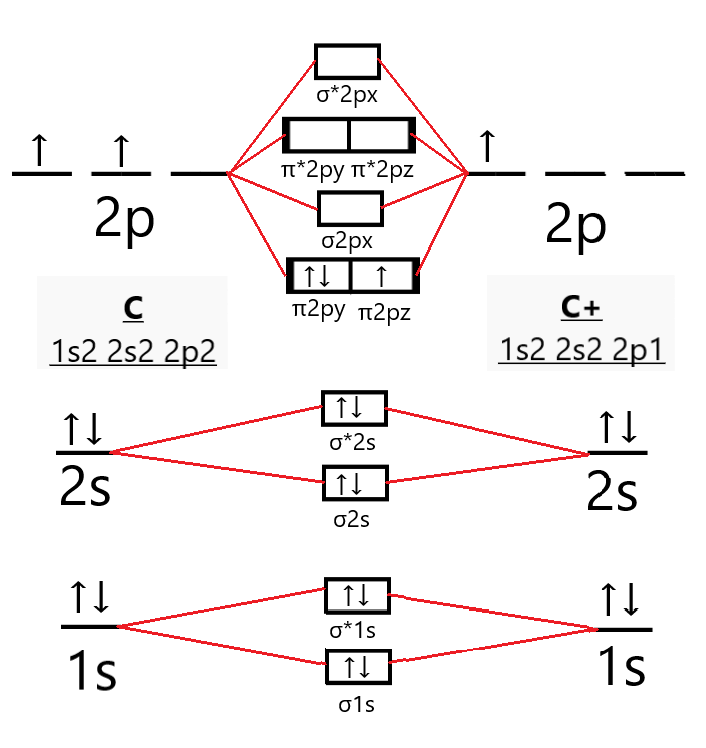
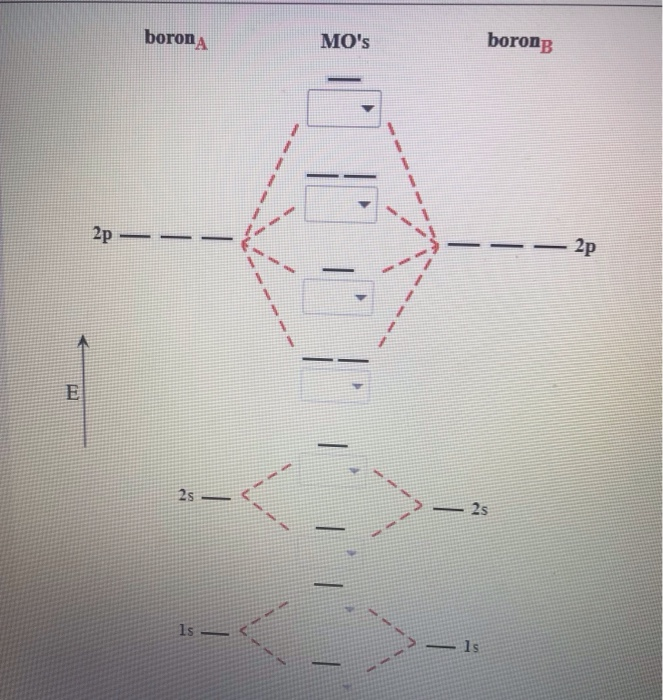
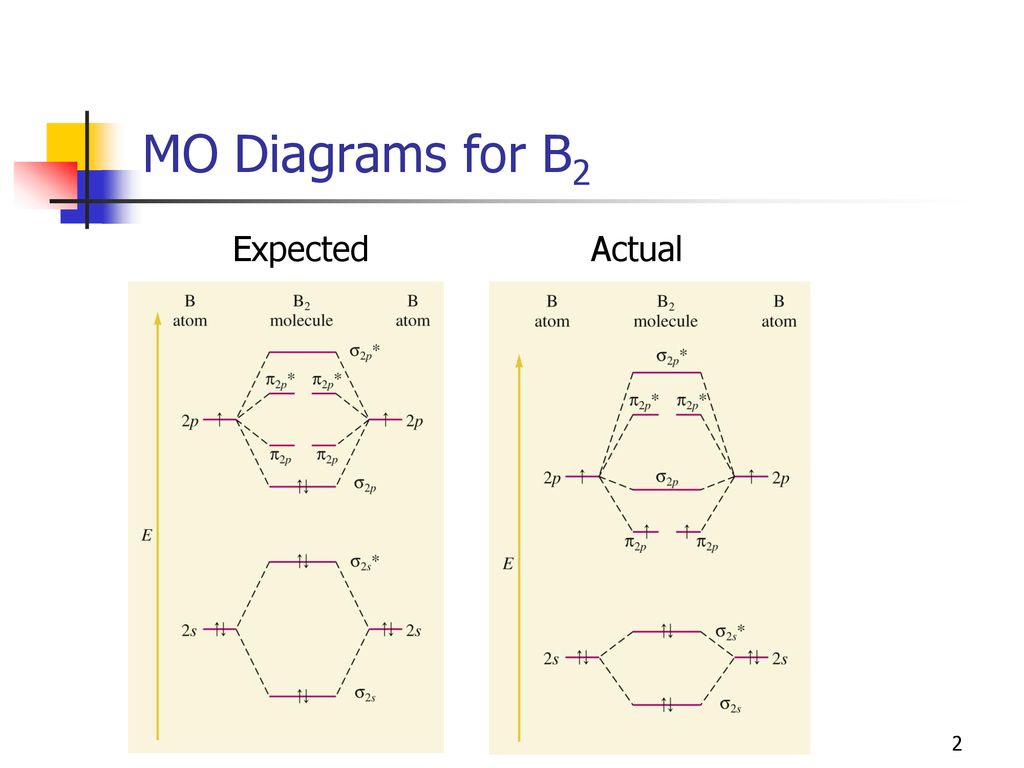
B2 Molecular Orbital Diagram. Collected from the entire web and summarized to include only the most important parts of it. Can be used as content for research and analysis. Home Blog Pro Plans Scholar Login. Advanced searches left . 3/3. Search only database of 12 mil and more summaries ... A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of ... The molecular orbital diagram for $\\ce{O2}$ says that the sigma 2p bonding molecular orbital is lower in energy than the pi 2p bonding molecular orbital. Why is this not the case in the $\\ce{B2}$ MO Jan 27, 2015 · Before we can draw a molecular orbital diagram for B₂, we must find the in-phase and out-of-phase overlap combinations for boron's atomic orbitals. Then we rank them in order of increasing energy. We can ignore the 1s orbitals, because they do not contain the valence electrons. Each boron atom has one 2s and three 2p valence orbitals. The 2s orbitals will overlap to form 2sσ and 2sσ ...
Nov 11, · As discussed in class the MO diagram for B 2 shows that it has two unpaired electrons (which makes it paramagnetic) and these electrons are in bonding molecular orbitals resulting in the equivalent bond strength of one bond. As discussed in class it is not a bond. What is the electron configuration, orbital diagram, and noble gas notation of potassium? Medium. View solution. > ... Women's Health may earn commission from the links on this page, but we only feature products we believe in. Why trust us? Wrap a band around a chinup bar so there is a loop under the bar. (Or use an assisted chinup machine.) Place your hand... Unlike vitamins A, D and C, “vitamin B” is actually a group of different vitamins, each of which has its own characteristics, function and side effects. Vitamin B2, more commonly known as riboflavin, is one such group. So, what are riboflav...
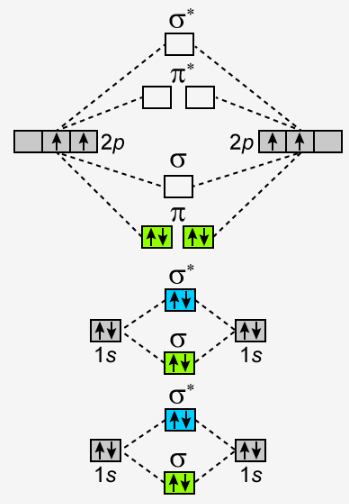
Molecular Orbital Theory allows us to predict the distribution of electrons within a molecule. This allows us to predict properties such as bond order, magnetism, and shape.. There are two types of MO diagrams:. Recall that the bonding MOs are those without an asterisk (e.g., σ 1s), while the antibonding MOs are those with an asterisk (e.g., σ 1s *).
Molecular Orbital (MO) Theory helps us to explain and understand certain Part B - Molecular Orbital Energy Diagrams & Bond Order . + and Be2.A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic ...
This video discusses how to draw the molecular orbital (MO) diagram for the B2 (boron) molecule. The bond order of the boron molecule is ...
The molecular orbital diagram for B 2 molecule is as follows: We know that bond order is the difference between the number of bonds and the antibonds. Now, we have to calculate the bond order of B 2 molecule using the formula as follows: Bond order = 1 2 ( Number of electrons in BMO) − ( Number of electrons in ABMO) From the diagram, we can ...
Riboflavin (Vitamin B2) received an overall rating of 8 out of 10 stars from 1 reviews. See what others have said about Riboflavin (Vitamin B2), including the effectiveness, ease of use and side effects. I got paranoid when my urine turn su...
This video shows the end of the Be2 molecule MO diagram and explains pi orbitals, paramagnetism, and the MO diagrams for B2.
N22+ B22+ B B2 CeV. Because of the difference in their atomic orbital energies, the 1s orbital of hydrogen and the 3s orbital of sulfur interact only weakly; this is shown in the diagram by a slight stabilization of the lowest energy molecular orbital with respect to the 3s orbital of sulfur. This lowest energy orbital is .
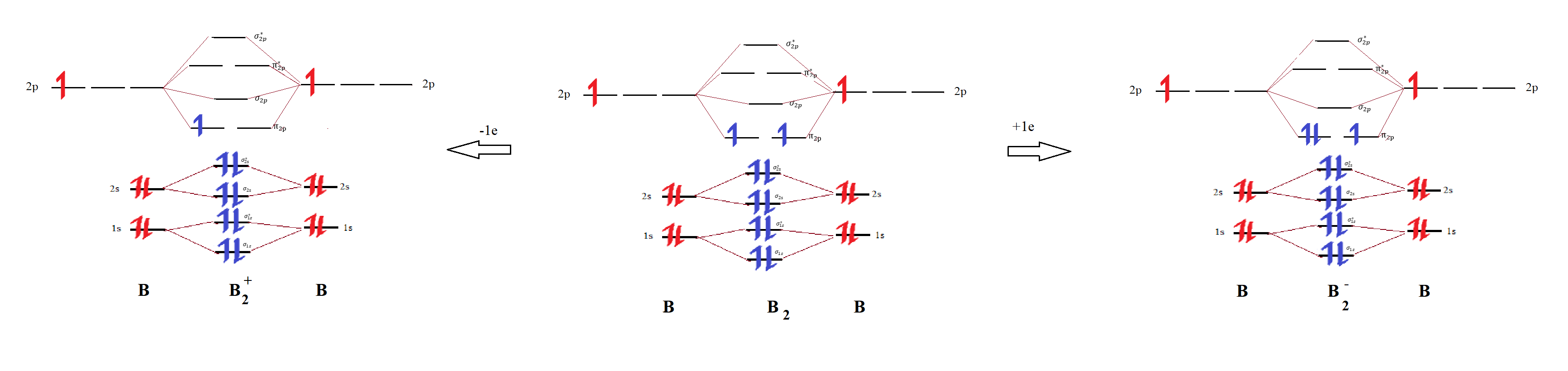
Draw the molecular orbital diagram for B2+ (this is not B-B. it has an electron missing, so its B-B cation!) The number of unpaired electrons in the B2+ molecule is 2 (two) 1 (one) 3 (three) zero ; Question: Draw the molecular orbital diagram for B2+ (this is not B-B. it has an electron missing, so its B-B cation!) The number of unpaired ...
MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine
1 So the bond order of B2 is equal to 1, which you can get by drawing the molecular orbital diagram and performing the equation Bond Order = . 5 * (# of bonding electrons - # of antibonding electrons). However, when you draw the Lewis structure of B2, you get a triple bond. Nov 11, 2016
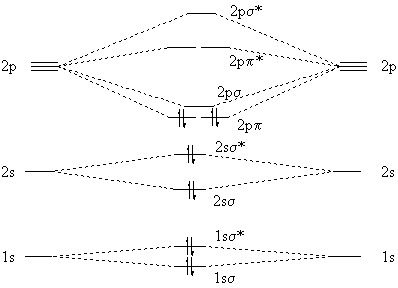
The orbital diagram for a diatomic molecule is. To find the bond order, add the 15 electrons in the molecular orbitals (the blue-colored energy levels in the diagram) one at a time until you have used them up. They completely fill all the orbitals except the highest-energy antibonding sigma 2p orbital.
Jan 26, 2015 · Jan 27, 2015 Before we can draw a molecular orbital diagram for B₂, we must find the in-phase and out-of-phase overlap combinations for boron's atomic orbitals. Then we rank them in order of increasing energy.
From the periodic table as we have already discussed the Molecular orbital diagrams of diatomic molecules of 1st two periods starting from Hydrogen to Neon. ...
Firstly the molecular orbital diagram is drawn then bond order is calculated using the bonding and antibonding electrons. After that, check for its magnetic ...
After reading the theory part draw the MO diagrams for the ... B2. C2. N2. O2. Ne2. F2 electron configuration. N. OF. OCCUPIED. MO. N. of bonding electrons1.



















0 Response to "41 molecular orbital diagram for b2"
Post a Comment