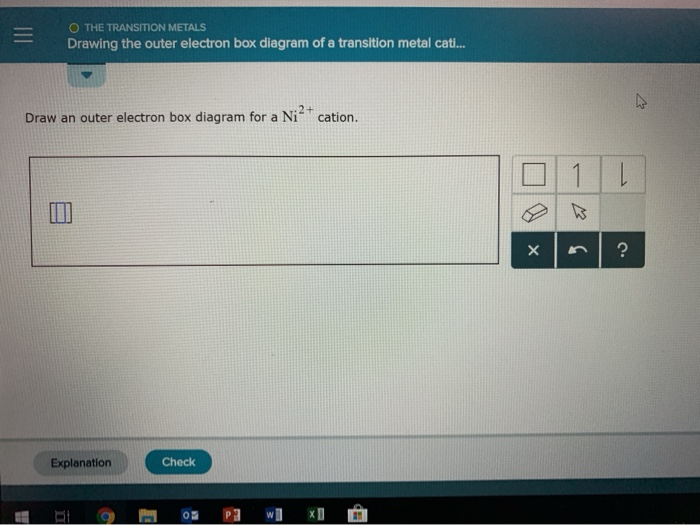
42 Draw An Outer Electron Box Diagram For A Cation.
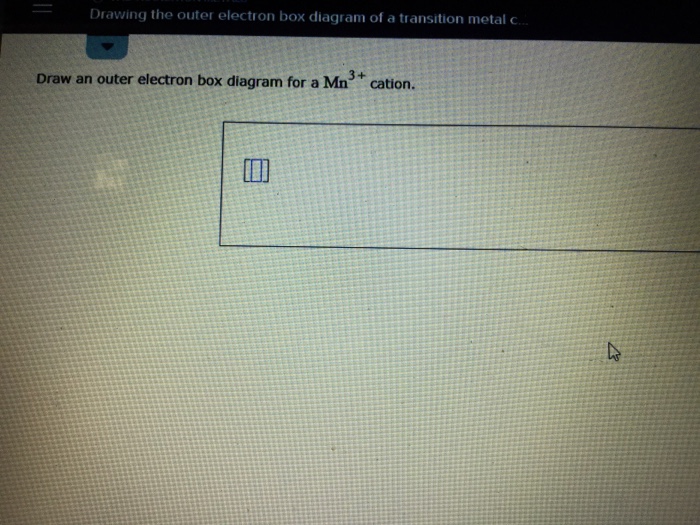
PDF Electron Configurations and Orbital Diagrams key Electron Configurations and Orbital Diagrams KEY Draw orbital diagrams for the following elements: 1. phosphorus ... Write the electron configuration (full, and in core notation) for the following ions: 1.-1Br +3 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 [Kr], [Ar] 3d 10 4s 2 4p 6 2. Sr +2 8. Draw an outer electron box diagram for a Nb^{3+} cation ... Question: Draw an outer electron box diagram for a N b3+ N b 3 + cation. D - block elements Generally, the electronic configuration of d block elements is (n−1)d1−10ns1−2 ( n − 1) d 1 − 10 n s 1 −...
diagram 2004 isuzu npr fuse box diagram 2004 isuzu nqr fuse box diagram 2013 freightliner cascadia fuse box diagram cat 6 wiring diagram pdf draw an outer electron box diagram for a rh3+ cation ej6 fuse box diagram et 500 gate motor wiring diagram ezgo txt steering box diagram jeep tj wiring diagram jeep wrangler wiring diagram free lnc 2000 wiring ...

Draw an outer electron box diagram for a cation.
What is the electron configuration of "Ti"^(2+)? | Socratic A good place to start when trying to figure out the electron configuration of an ion is the electron configuration of the neutral parent atom.. In this case, titanium, #"Ti"#, is located in period 4, group 4 of the periodic table and has an atomic number of #22#. This means that a neutral titanium atom will contain #22# protons in its nucleus and #22# electrons surrounding its nucleus. what is the electron dot structure for calcium ... The main requirement to draw the electron dot structure is the valence electrons. Accordingly, the valence electron of carbon and chlorine are 4 and 7 respectively. What do you use a dot diagram for? A dot diagram, also called a dot plot, is a statistical chart consisting of data points plotted on a fairly simple scale. 6.4 Electronic Structure of Atoms (Electron Configurations ... Write the electron structure of the two cations. Thallium was used as a poison in the Agatha Christie mystery story "The Pale Horse." Thallium has two possible cationic forms, +1 and +3. The +1 compounds are the more stable. Write the electron structure of the +1 cation of thallium. Write the electron configurations for the following atoms ...
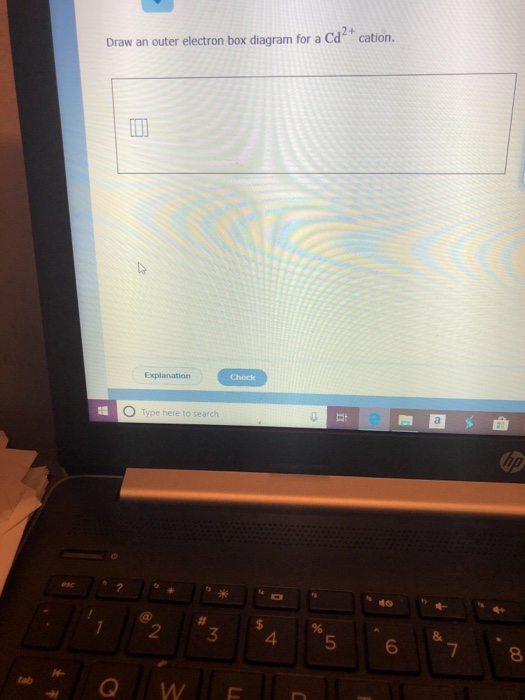
Draw an outer electron box diagram for a cation.. Draw an outer electron box diagram for a [{MathJax ... Draw an outer electron box diagram for a M o2+ M o 2 + cation. Transition Elements In the periodic table, elements are classified on the basis of their electronic configuration meaning the... Solved Draw an outer electron box diagram for a Rh cation ... Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Rh2+ have 43 electrons. It's …. View the full answer. Transcribed image text: Draw an outer electron box diagram for a Rh cation. What is the electron configuration of Cd2+? | Socratic ["Kr"]4d^10 Your starting point here will be the electron configuration of a neutral cadmium atom. Cadmium, "Cd", is located in period 5, group 12 of the periodic table and has an atomic number equal to 48. This means that a neutral cadmium atom will have a total of 48 electrons surrounding its nucleus. This also tells you that the "Cd"^(2+) cation, which has two electrons less than the ... 8.2c Drawing a box diagram of the electron configuration ... About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ...
Solved 4+ Draw an outer electron box diagram for a Cr ... This problem has been solved! Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Transcribed image text: 4+ Draw an outer electron box diagram for a Cr* cation. Solved Draw an outer electron box diagram for a Tc cation ... This problem has been solved! Draw an outer electron box diagram for a Tc3+ cation. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Transcribed image text: Draw an outer electron box diagram for a Tc cation. Why is Ru3+ electron configuration [Kr] 4d... | Clutch Prep We're being asked to determine why is the Ru 3+ electron configuration [Kr] 4d 5 5s 0 instead of having a full s orbital.. Before we can do that, we have to first write the electron configuration of a neutral ground state Ruthenium (Ru).. You can determine the ground-state electron configuration of Ru by locating the position Ru in the periodic table.. Ground-state means that the element is ... Electron Configurations & Orbital Diagrams - www ... Orbital box diagrams are the electron configuration problems where you have to draw each electron out as a little arrow inside a box. Hund's Rule tells you the electrons don't like roommates, and the Pauli Exclusion Principle tells you that if an electron has to have a roommate, it wants one with opposite spin.
diagram 1756-ob32 wiring diagram circuit 2007 suzuki xl7 fuse box diagram 2017 mini cooper fuse box diagram cpu1215c wiring diagram detroit diesel one box diagram draw an outer electron box diagram for a nb4+ cation embsay signal box diagram ford explorer fuse box diagram gamewell zans 400 wiring diagram golf cart battery meter wiring diagram ht12e ... Drawing Ionic Bonds Worksheet - worksheet Write the electron dot structure lewis dot structure for covalent compounds or ions. The c c bonds in c 3 h 8 nonpolar covalent ii. For each of the following ionic bonds. Draw an arrow or more if needed to show the transfer of electrons to the new element. Sodium nitride na3n 60 1. Draw a lewis dot structure for the valence shell of each element. Electron Configurations, Orbital Box Notation (M7Q7 ... Electron Configuration Exceptions. The periodic table can be a powerful tool in predicting the electron configuration of an element. However, we do find exceptions to the order of filling of orbitals shown in Figure 3 and Figure 4.For instance, the electron configurations (shown in Figure 6) of the transition metals chromium (Cr; atomic number 24) and copper (Cu; atomic number 29), among ... Electron configuration for Ru3+. - Clutch Prep Our videos prepare you to succeed in your college classes. Let us help you simplify your studying. If you are having trouble with Chemistry, Organic, Physics, Calculus, or Statistics, we got your back! Our videos will help you understand concepts, solve your homework, and do great on your exams.
Aleks Drawing a box diagram of the electron configuration ... About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ...
Electron Configuration of Transition Metals - Chemistry ... Transition Metals with an Oxidation State. In the ground state, the electron configuration of the transition metals follows the format, ns 2 nd x.As for the electron configuration for transition metals that are charged (i.e. Cu +), the electrons from the s orbital will be moved to the d-orbital to form either ns 0 nd x or ns 1 nd x.. It is helpful to first write down the electron configuration ...
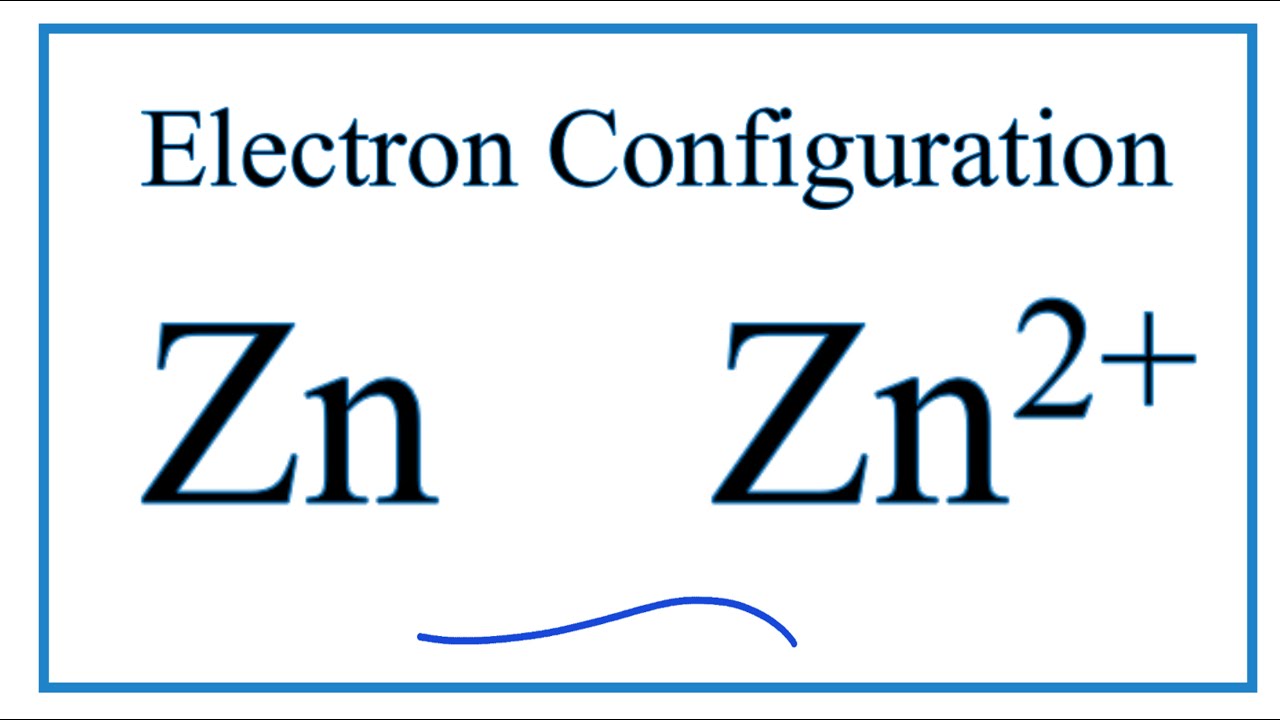
What is the electron configuration for Zn2+? | Socratic The electron configuration of Zn2+ is 1s22s22p63s23p63d10. Zinc is a d-block element, also known as a transition element. For the d-block elements, the outermost s-sublevel has higher energy than the d-sublevel, which is contrary to what the Aufbau diagram indicates. When d-block elements lose electrons, they lose the highest energy s electrons ...
Draw the orbital diagram for ion Co 2 ... - Clutch Prep Q. Draw the orbital diagrams (box/line notation) for the following species:Mn2+Cu See all problems in The Electron Configuration: Ions Frequently Asked Questions
How to Do Orbital Diagrams - Sciencing Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics.
How do you write the electron configuration for Ni^(+2 ... Nickel # Ni_28 # has 28 electrons. 18 electrons fill up the third electron shell leaving 10 valance electrons. 2 electrons in the 4s and 8 elections in the 3d. When Nickel becomes #Ni^+2# Nickel has lost 2 electrons leaving the atom with only 8 valance electrons.. The 4s electrons a lower energy level that the 3d electrons because of the simpler electron path of the S orbital.
Electronic Structure of Atoms (Electron Configurations ... For orbital diagrams, this means two arrows go in each box (representing two electrons in each orbital) and the arrows must point in opposite directions (representing paired spins). The electron configuration and orbital diagram of helium are: The n = 1 shell is completely filled in a helium atom.
Orbital Diagram For Fe3+ Transition Fe3+ ions and draw the orbital box diagrams for both ions. Using this. There for 1s2 2s2 2p6 3s2 3p6 3d5 is the electronic configration for Fe3+. half of electrons (there must be one electron in each orbital, and d has 5 orbitals). That's for filling up orbitals for ground state atoms.
6.4 Electronic Structure of Atoms (Electron Configurations ... Write the electron structure of the two cations. Thallium was used as a poison in the Agatha Christie mystery story "The Pale Horse." Thallium has two possible cationic forms, +1 and +3. The +1 compounds are the more stable. Write the electron structure of the +1 cation of thallium. Write the electron configurations for the following atoms ...
what is the electron dot structure for calcium ... The main requirement to draw the electron dot structure is the valence electrons. Accordingly, the valence electron of carbon and chlorine are 4 and 7 respectively. What do you use a dot diagram for? A dot diagram, also called a dot plot, is a statistical chart consisting of data points plotted on a fairly simple scale.
What is the electron configuration of "Ti"^(2+)? | Socratic A good place to start when trying to figure out the electron configuration of an ion is the electron configuration of the neutral parent atom.. In this case, titanium, #"Ti"#, is located in period 4, group 4 of the periodic table and has an atomic number of #22#. This means that a neutral titanium atom will contain #22# protons in its nucleus and #22# electrons surrounding its nucleus.
![Give the ground state electron configuration for Br -.(A) [Ar]4s23d104p5(B) [Ar] 4s24p6(C) [Ar]4s24p6(D) [Ar]4s24d104p4(E) [Ar]4s23d104p6](https://cdn.clutchprep.com/video_thumbnails/34471.jpg)
Give the ground state electron configuration for Br -.(A) [Ar]4s23d104p5(B) [Ar] 4s24p6(C) [Ar]4s24p6(D) [Ar]4s24d104p4(E) [Ar]4s23d104p6








/800px-Orbital_representation_diagram.svg-589bd6285f9b58819cfd8460.png)







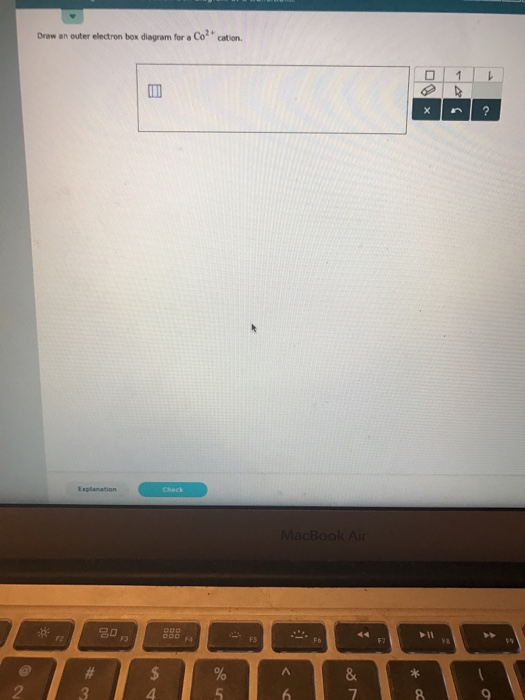










0 Response to "42 Draw An Outer Electron Box Diagram For A Cation."
Post a Comment