39 mo diagram of b2
molecular orbital theory - Why is diboron (B2 ... The resulting diagram looks (in approximation) like this: If you now fill the three valence electrons of each boron into the diagram according to Hund's rule, you will see that each π orbital will get one electron. This results in a triplet ground state. The finished valence molecular orbital diagram is pictured below. C22- Molecular Orbital Diagram B2 MO diagram with no sp mixing: B2 = 6 e⁻. Label the sigma bonding molecular orbitals on the diagram above using the designation. d. Give the electron configurations for the species C2 and C When we draw the C2 MO, we have everything up till the PiPy Orbitlal filled, and the next orbital tht would be filled would be the sigma2Pz.
What is the molecular orbital diagram for B_2? | Socratic Jan 27, 2015 Before we can draw a molecular orbital diagram for B₂, we must find the in-phase and out-of-phase overlap combinations for boron's atomic orbitals. Then we rank them in order of increasing energy. We can ignore the 1s orbitals, because they do not contain the valence electrons. Each boron atom has one 2s and three 2p valence orbitals.
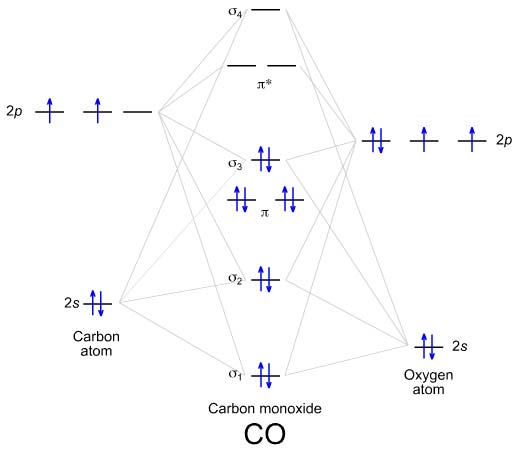
Mo diagram of b2
Solved 3) 10 points The MO diagram below is appropriate ... 3) 10 points The MO diagram below is appropriate for B2. Based on this diagram, a) draw the molecular orbital filling diagram for B2 b) what is the bond order of B2? c) is B2 diamagnetic or paramagnetic? π2p σ2 s 4) 15 points Calculate the lattice energy for MgO (s) using a Born-Haber cycle and the fol information: Net eneroy change is 6019. M.O. Diagram for B2 - CHEMISTRY COMMUNITY As discussed in class the MO diagram for B 2 shows that it has two unpaired electrons (which makes it paramagnetic) and these electrons are in bonding molecular orbitals resulting in the equivalent bond strength of one bond. As discussed in class it is not a bond. Draw the molecular orbital diagram for:(i) Be2(ii) B2 and ... MO electronic configuration: Bond order: Here Nb = 4, Na = 2 Bond order = The two boron atom is B2 molecules are linked by one covalent bond. Magnetic properties: Since each 2p x and 2p y MO contains unpaired electron, therefore B 2 molecule is paramagnetic.
Mo diagram of b2. following diatomic omonuclear molecules: H2, B2, C2, N2, O2 ... Problem 1. After reading the theory part draw the MO diagrams for the following diatomic omonuclear molecules: H2, B2, C2, N2, O2, Ne2, F2.13 pages Answered: Hint Resources 2671/3100 2s Identify ... - bartleby Hint Resources 2671/3100 2s Identify the MO diagram for B,. B2 valence e : O diagram A Odiagram B Identify the MO diagram for C, C, valence e : diagram diagram B Identify the MO diagram for N, N, valence e : diagram A Odiagram B Identify the MO diagram for O, O2 valence e-: diagram A O diagram B Identify the MO diagram for F2 F, valence e : diagram A diagram B SAMSUNG $ % & 4 6 t r i y u O j k ... B2 Molecular Orbital Diagram - Summarized by Plex.page ... B2 Molecular Orbital Diagram. Collected from the entire web and summarized to include only the most important parts of it. Can be used as content for research and analysis. Home Blog Pro Plans Scholar Login. Advanced searches left . 3/3. Search only database of 12 mil and more summaries ... Solved Draw the molecular orbital diagram for B2+ (this is ... Chemistry questions and answers. Draw the molecular orbital diagram for B2+ (this is not B-B. it has an electron missing, so its B-B cation!) The number of unpaired electrons in the B2+ molecule is 2 (two) 1 (one) 3 (three) zero. Question: Draw the molecular orbital diagram for B2+ (this is not B-B. it has an electron missing, so its B-B cation!)
According to the molecular orbital theory, what is the ... "BO" = 1/2 Boron atom is atomic number 5 in the periodic table, so it has five electrons. Thus, B_2 carries ten total electrons. The atomic orbitals each boron contributes consists of the 1s, 2s, and 2p. The ns orbitals combine to give a portion of the molecular orbital (MO) diagram like this: where sigma^"*" indicates an antibonding sigma (sigma) MO, and sigma is the bonding MO. Which of the following is paramagnetic? (use the mole... Our videos prepare you to succeed in your college classes. Let us help you simplify your studying. If you are having trouble with Chemistry, Organic, Physics, Calculus, or Statistics, we got your back! Our videos will help you understand concepts, solve your homework, and do great on your exams. How to Make the Molecular Orbital Diagram for B2 (Bond ... This video discusses how to draw the molecular orbital (MO) diagram for the B2 (boron) molecule. The bond order of the boron molecule is also calculated and ... PDF MO Diagrams for Linear and Bent Molecules Build MO diagram. We expect six MOs, with the O 2pytotally nonbonding. Water H He Li Be B C N O F Ne B C N O F Ne Na Mg Al Si P S Cl Ar Al Si P S Cl Ar 1s 2s 2p 3s 3p -13.6 eV -15.8 eV -32.4 eV Based on the large ΔE, we expect O 2s to be almost nonbonding. Water With the orbital shapes, symmetries, and energies in hand we can make the MO ...
Be2 Molecular Orbital Diagram - schematron.org Nov 11, · As discussed in class the MO diagram for B 2 shows that it has two unpaired electrons (which makes it paramagnetic) and these electrons are in bonding molecular orbitals resulting in the equivalent bond strength of one bond. As discussed in class it is not a bond. PDF MO Diagrams for Diatomic Molecules MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine MO Diagrams - GitHub Pages Draw out the MO diagram and label in the valence electrons. Boron has 2 electrons in the `2s` orbitals and 1 electron in the `2p` orbital. That's it for the MO diagram of `B_2`! To check, count how many electrons there are in total. `B_2` has `2(3)=6` valence electrons. The MO diagram has `6` electrons as well. What Is The Bond Order Of B2 - questionfun.com Hey guys, So the bond order of B2 is equal to 1, which you can get by drawing the molecular orbital diagram and performing the equation Bond Order = . 5 * (# of bonding electrons - # of antibonding electrons). However, when you draw the Lewis structure of B2, you get a triple bond . (Visited 21 times, 1 visits today)
PDF MO Diagram for B Through N - MhChem CH 222 MO Diagram for B2 Through N2 Created Date: 20160427212553Z ...
What is the molecular orbital diagram for B2? - Clutch Prep There are two types of MO diagrams: Recall that the bonding MOs are those without an asterisk (e.g., σ1s), while the antibonding MOs are those with an asterisk (e.g., σ1s*). 80% (373 ratings) View Complete Written Solution Problem Details What is the molecular orbital diagram for B2?
Molecular Orbital Diagram Be2 Molecular Orbital Diagram Be2. Even rather simple molecular orbital (MO) theory can be used to predict which we start reading from the bottom of the diagram because this is how MO diagrams are constructed, Diberyllium, Be2, has a bond order of zero and is unknown. From the above MO diagram we can see that number of elctrons in the bonding and ...
Molecular Orbital Theory - MIT OpenCourseWare Draw a molecular orbital diagram and determine the bond order expected for the molecule B. 2. For full credit on MO diagrams, • label increasing energy with an arrow next to the diagram. • pay attention to whether the question asks for valence electrons or all electrons. • for any bonding orbital drawn, include the corresponding anti ...
Use MO diagrams to place B2+, B2, and B2- in order of (a ... Use MO diagrams to place B2+, B2, and B2- in order of (a) decreasing bond energy; (b) decreasing bond length. Explanation. The electron configuration of . B B B. atom is . 1 s 2 2 s 2 2 p 1 1s^2 \\ 2s^2 \\ 2p^1 1 s 2 2 s 2 2 p 1. At first, build the molecular orbital diagram for .
Draw MOT diagram for B2 molecule and calculate its class ... The molecular orbital diagram for B 2 molecule is as follows: We know that bond order is the difference between the number of bonds and the antibonds. Now, we have to calculate the bond order of B 2 molecule using the formula as follows: Bond order = 1 2 ( Number of electrons in BMO) − ( Number of electrons in ABMO) From the diagram, we can ...
Molecular Orbital Diagram MO Electron Configuration B2 ... Transcribed image text : 5. Using a molecular orbital (MO) energy diagram, indicate the bond order of the following: B2 C2- Liz MO Diagram MO Diagram MO Diagram Bond Order Bond Order Bond Order Eur] = 3 = 1 20 1216-4] = 2 2 = 1 6. Using a...
Diatomic Species | MO theory | Chemogenesis Diatomic Species by Molecular Orbital Theory. Even rather simple molecular orbital (MO) theory can be used to predict which homonuclear diatomic species - H 2, N 2, O 2, etc. - will exist, explain many properties - for example why O 2 is a paramagnetic diradical - and identify the important frontier molecular orbitals (FMOs).
molecular orbital diagram of B2 - Brainly.in Molecular orbital diagram of B2 1 See answer manfoosah2002 is waiting for your help. Add your answer and earn points. priyesharma02 priyesharma02 Molecular orbita diagram of B2 New questions in Chemistry. ejj-bgir-qdr hajqjqbwvgwhw short note on all the types of Quantum numbers.
Br2 Lewis Structure, Molecular Geometry, Hybridization ... The MO diagram or Molecular Orbital diagram is an extension of the 3-dimensional molecular design and gives a better understanding of the structure of an atom. Molecular Diagram also reflects upon bond length, bond shape, bond energy, and the bond angle between 2 atoms. Br2 is a simple compound as it is formed by 2 atoms of the same element.
Molecular Orbital Theory. B2 - YouTube This video shows the end of the Be2 molecule MO diagram and explains pi orbitals, paramagnetism, and the MO diagrams for B2.
Molecular Orbital Diagram Be2 - schematron.org + and Be2.A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular. 1.
Draw the molecular orbital diagram for:(i) Be2(ii) B2 and ... MO electronic configuration: Bond order: Here Nb = 4, Na = 2 Bond order = The two boron atom is B2 molecules are linked by one covalent bond. Magnetic properties: Since each 2p x and 2p y MO contains unpaired electron, therefore B 2 molecule is paramagnetic.
M.O. Diagram for B2 - CHEMISTRY COMMUNITY As discussed in class the MO diagram for B 2 shows that it has two unpaired electrons (which makes it paramagnetic) and these electrons are in bonding molecular orbitals resulting in the equivalent bond strength of one bond. As discussed in class it is not a bond.
Solved 3) 10 points The MO diagram below is appropriate ... 3) 10 points The MO diagram below is appropriate for B2. Based on this diagram, a) draw the molecular orbital filling diagram for B2 b) what is the bond order of B2? c) is B2 diamagnetic or paramagnetic? π2p σ2 s 4) 15 points Calculate the lattice energy for MgO (s) using a Born-Haber cycle and the fol information: Net eneroy change is 6019.

0 Response to "39 mo diagram of b2"
Post a Comment