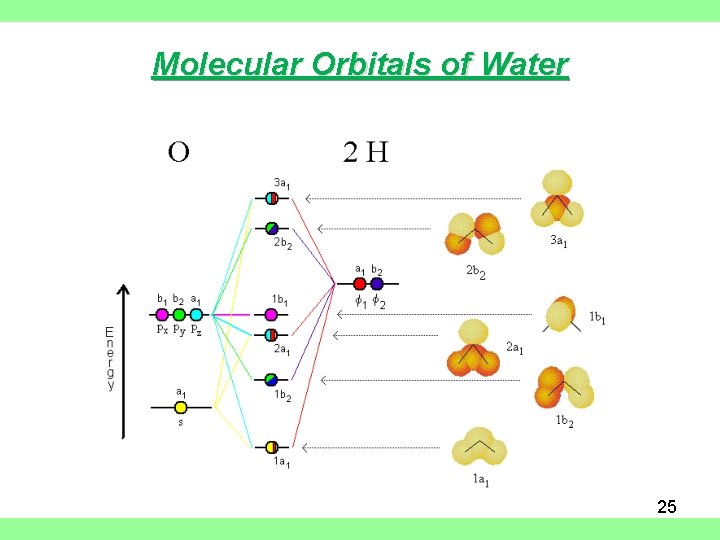
39 molecular orbital diagram for water
PDF Molecular Orbitals in | 9-2 Molecular Orbital Energy Level Diagrams Molecular orbital theory accounts for the fact that O2 has two unpaired elec-trons. This ability of MO theory to explain the paramagnetism of O2 gave it Heteronuclear Diatomic Species The following is a molecular orbital energy level diagram for a heteronuclear diatomic molecule, XY, in which both X... Molecular Orbitals: Molecular Orbital Theory | SparkNotes Figure %: Orbital correlation diagram for homonuclear diatomic molecules other than B2, C2, and N2. To draw the correlation diagrams for heteronuclear diatomic molecules, we face a new problem: where do we place the atomic orbitals on an atom relative to atomic orbitals on other atoms?
English: Molecular orbital diagram for water. Firefly 8.2.0 DFT... File:Molecular Orbitals for Water.png. From Wikimedia Commons, the free media repository. Jump to navigation Jump to search. DescriptionMolecular Orbitals for Water.png. English: Molecular orbital diagram for water.

Molecular orbital diagram for water
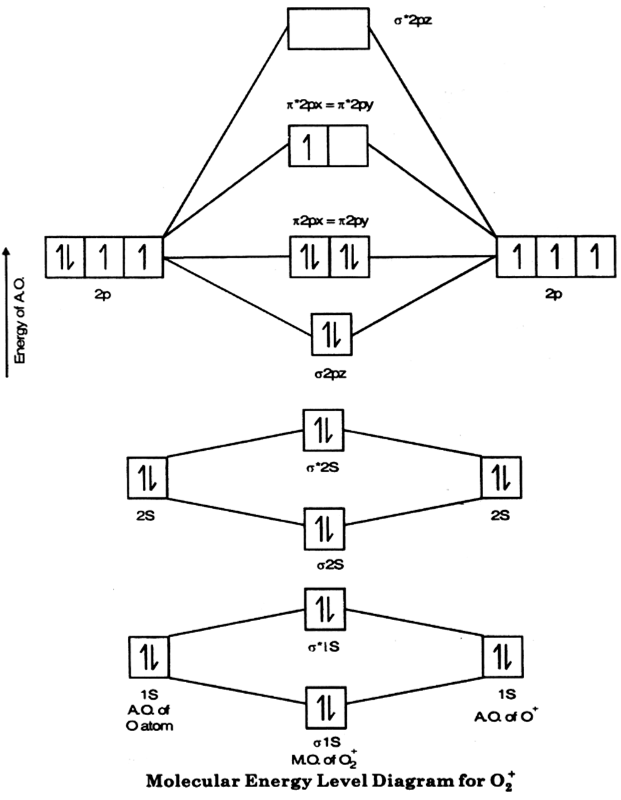
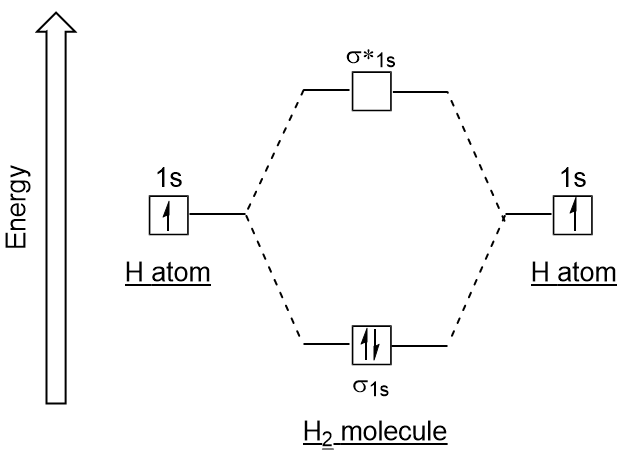
Water molecular orbital diagram - Big Chemical Encyclopedia Assembly of the molecular orbital diagram for water C2y point group) using symmetry-adapted orbitals on the ligands and the central atom/ orbitals. Theoretical ionization potentials and molecular orbital diagrams obtained from 3-2IG SCF calculations are also given. Asked for: molecular orbital energy-level diagram, valence electron... : Molecular Orbital Energy-Level Diagram for H 2. The two available electrons (one from each H atom) in this diagram fill the bonding σ 1 s molecular orbital. Because the energy of the σ 1 s molecular orbital is lower than that of the two H 1 s atomic orbitals, the H 2 molecule is more stable... Energy level diagram for Molecular orbitals - Chemical Bonding and... 3) If Nb = Na ,the molecule is again unstable because influence of electrons in the antibonding molecular orbital is greater than the bond influence of electron in the bonding molecular orbitals. Diagram for O2+ is wrong because 2p atomic orbital of 2nd O atom will have only 3 e
Molecular orbital diagram for water. Molecular Orbital Theory | Boundless Chemistry Molecular orbital diagrams are diagrams of MO energy levels, shown as short horizontal lines in the center. A compound 's empirical formula is the simplest integer ratio of its constitutional chemical elements. For example, water is always composed of a 2:1 ratio of hydrogen to oxygen atoms. Molecular Orbital Theory (5.4) - Chemistry 110 Water, like most molecules, contains all paired electrons. Draw the molecular orbital diagram for the oxygen molecule, O2. From this diagram, calculate the bond order for O2. Creating molecular orbital diagrams for molecules with more than two atoms relies on the same basic ideas as the... Newest 'molecular-orbital-theory' Questions - Chemistry Stack... Use of molecular orbital (MO) theory allows for an understanding of the observed properties (shape, reactivity) of molecules. The tag should be applied to all questions related to MO theory, from questions about the qualitative use of the theory to questions about the underlying quantum... Solved: Hybrid Orbitals: Complete The MO Diagram Of Water ... In this problem, you will build a hybrid molecular orbital diagram for water. Next, place the molecular orbitals formed by interaction of these AOs in the middle column (labeled "H2O") with correct orbital labels and occupy them with the correct number of electrons.
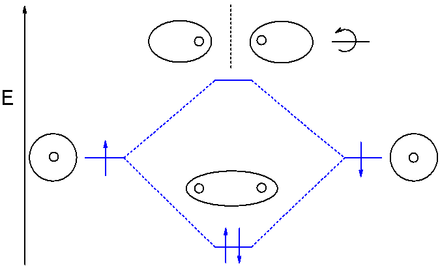
Molecular Orbital (MO) Diagram of Polyatomic molecules Beryllium... In polyatomic molecules we can have more than two atoms combining, e.g. in case of beryllium hydride there are 3 atoms overlapping simultaneously. So in... What is the molecular orbital diagram of O2 and F2? - Quora The orbital diagram for a diatomic molecule is. To find the bond order, add the 15 electrons in the molecular orbitals (the blue-colored energy levels in the diagram) one at a time until you have used them up. They completely fill all the orbitals except the highest-energy antibonding sigma 2p orbital. MO Diagrams | Molecular Orbital Diagram Maker A bare molecular orbital diagram is presented and you must drag the correct orbitals and labels onto the diagram. The diagram is then completed by filling the energy levels with the correct number of electrons. The following molecules are currently available: Molecules of the First Row Molecular Orbital Theory Molecular orbital theory is more powerful than valence-bond theory because the orbitals reflect the The bonding molecular orbital concentrates electrons in the region directly between the two nuclei. The molecular orbital diagram for an O2 molecule would therefore ignore the 1s electrons on both...
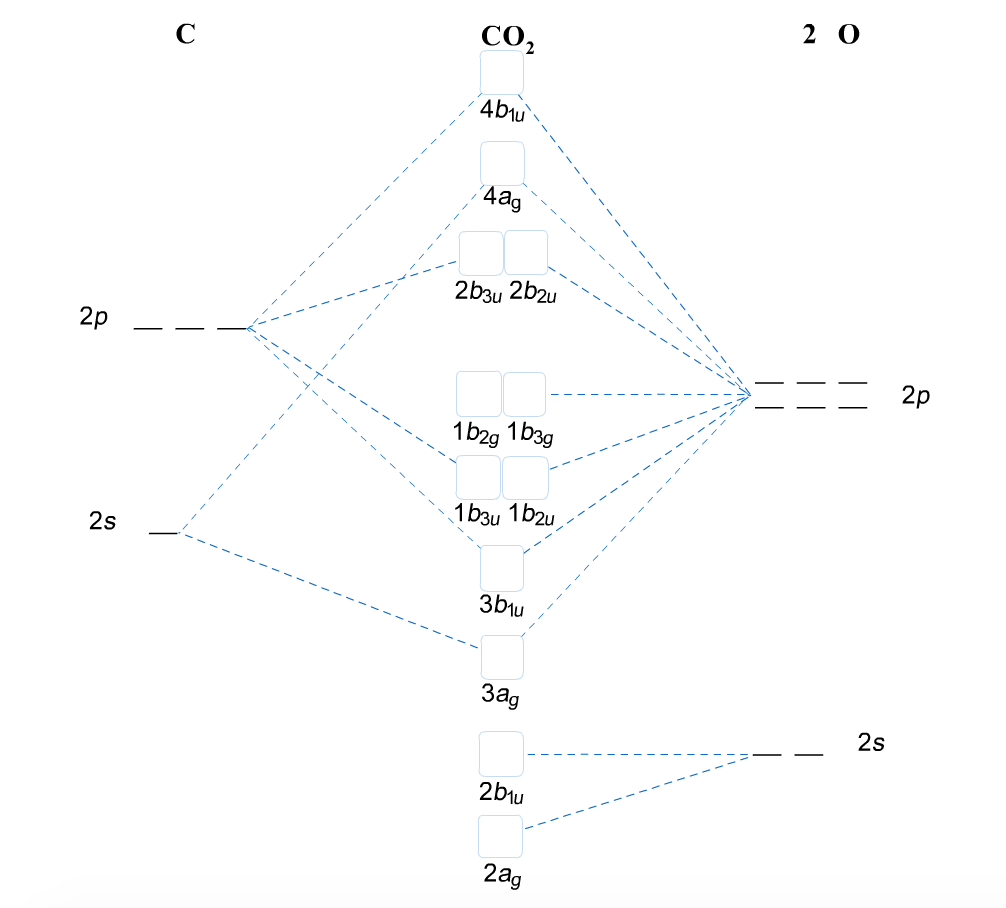
Molecular Orbital Diagrams, Bond Order, and Number of Unpaired... Water, like most molecules, contains all paired electrons. Living things contain a large percentage of water, so they demonstrate diamagnetic behavior. Figure 8. This is the molecular orbital diagram for the homonuclear diatomic Be2+, showing the molecular orbitals of the valence shell only. Molecular Orbital Theory: Explanation, Illustrations and... - Embibe Molecular Orbital Theory: To simplify things, we will consider the interaction of the orbitals containing valence electrons to create molecular orbitals. Have you ever thought about how sigma and pi bonds are formed? What is the difference between diamagnetic and paramagnetic behaviour? Molecular orbital diagram of water - Brainly.in Mixing takes place between same-symmetry orbitals of comparable energy resulting a new set of MO's for water: 2a1 MO from mixing of the oxygen 2s AO and the hydrogen σ MO. Molecular Orbital diagrams of polyatomic species In the diatomic species, in constructing the molecular orbital diagram, we simply show the energies of the atomic orbitals of the two species on either These basis orbitals are hybrid orbitals of the atoms, and reflect the symmetry of the molecule. A simple MO diagram for water is as shown in the table.
Introduction to Inorganic Chemistry/Molecular Orbital Theory... Valence bond (VB) theory gave us a qualitative picture of chemical bonding, which was useful for predicting the shapes of molecules, bond strengths, etc. It fails to describe some bonding situations accurately because it ignores the wave nature of the electrons.
Introduction to Molecular Orbital Theory | Water In the water molecule the highest occupied orbital, (1b1) is non-bonding and highly localized on the oxygen atom, similar to the non-bonding orbitals The atomic orbitals combine to produce the following molecular orbital diagram: Comparison of the above energy level diagram wit hthat for nitrogen...
8.4 Molecular Orbital Theory - Chemistry 2e | OpenStax Water, like most molecules, contains all paired electrons. The filled molecular orbital diagram shows the number of electrons in both bonding and antibonding molecular orbitals. Draw the molecular orbital diagram for the oxygen molecule, O2. From this diagram, calculate the bond...
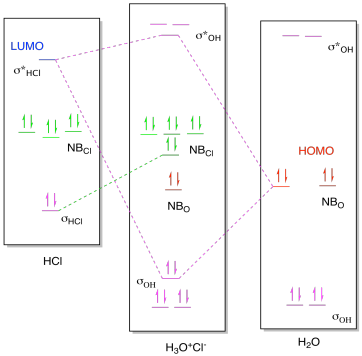
PDF Microsoft Word - Chapter 1_6_SY.doc Molecular Orbital Diagrams (Heteronuclear Diatomics) The molecular orbital diagram for the heteronuclear diatomic compound nitrogen monoxide Water vapor pressure varies with temperature. Table 10.X shows vapor pressure values for moderate temperatures; a more complete table is found...
Tutorial on Chemical Bonding, Part 8 of 10 (Molecular orbitals) The molecular orbital model is by far the most productive of the various models of chemical bonding, and serves as the basis for most quantiative calculations, including those that Construct a "molecular orbital diagram" of the kind shown in this lesson for a simple diatomic molecule, and indicate...
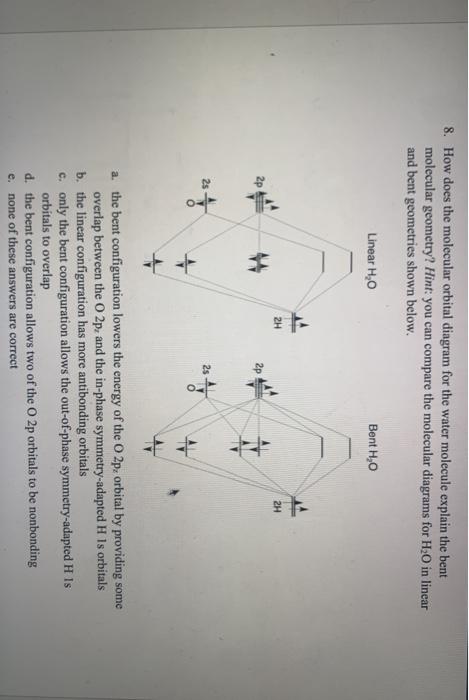
PDF Microsoft PowerPoint - Polyatomic Molecular Orbital Theory... Why is Water Bent??? 9. Molecular Orbital Theory - linear XH2 molecules. The Walsh diagram shows what happens to the molecular orbitals for a set of molecules which are related in structure. In this case, the difference is the H-X-H bond angle which decreases from 180o to 90o.
Molecular Orbital Diagram: study guides and answers on Quizlet Draw a molecular orbital diagram for He2, calculate the bond order and determine its stability. Show the shapes of the molecular orbitals also. Select one: a. Argon is a fairly inert substance b. Water is a liquid at room temperature c. Sodium reacts with water to form sodium hydroxide and hydrogen gas...
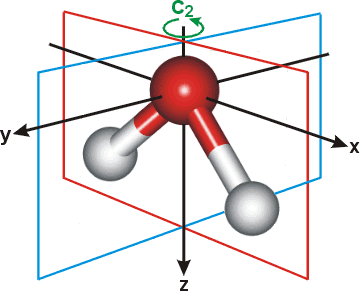
PDF Character Tables | Bonding in the Water Molecule The water HOMO has B1 symmetry The water HOMO is a pure oxygen 2px orbital and does not have any contribution from H This lone-pair orbital is orthogonal to the molecular plane and is responsible for the basic/nucleophilic character MO Diagram for the Water Molecule. 5.03 Inorganic Chemistry.
PDF Microsoft PowerPoint - An introduction to Molecular Orbital Theory.ppt... - MO diagrams for Inorganic complexes. 2. Lecture 1 Lecture 2. • It is a waste of both the lecturers and students time if the tutorial to ends up being a lecture covering questions. 5. An introduction to Molecular Orbital Theory.
Molecular Orbital Theory: Energy level diagram for molecular orbitals Molecular orbital theory was put forward by Hund and Mullikan in 1932. This theory is modern and more rational. This theory assume that in molecules, atomic orbitals lose their identity and the electrons in molecules are present in new orbitals called molecular orbitals.
Molecular orbital diagram - Wikipedia A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular.
PDF Procedure for Constructing Molecular Orbital Diagrams Based on... Last time you learned how to construct molecule orbital diagrams for simple molecules based on the symmetry of the atomic orbitals. We could use the symmetry-based method to construct molecular orbital diagrams for larger molecules as well, but this can get complicated for larger structures.
Energy level diagram for Molecular orbitals - Chemical Bonding and... 3) If Nb = Na ,the molecule is again unstable because influence of electrons in the antibonding molecular orbital is greater than the bond influence of electron in the bonding molecular orbitals. Diagram for O2+ is wrong because 2p atomic orbital of 2nd O atom will have only 3 e
Asked for: molecular orbital energy-level diagram, valence electron... : Molecular Orbital Energy-Level Diagram for H 2. The two available electrons (one from each H atom) in this diagram fill the bonding σ 1 s molecular orbital. Because the energy of the σ 1 s molecular orbital is lower than that of the two H 1 s atomic orbitals, the H 2 molecule is more stable...
Water molecular orbital diagram - Big Chemical Encyclopedia Assembly of the molecular orbital diagram for water C2y point group) using symmetry-adapted orbitals on the ligands and the central atom/ orbitals. Theoretical ionization potentials and molecular orbital diagrams obtained from 3-2IG SCF calculations are also given.




















0 Response to "39 molecular orbital diagram for water"
Post a Comment