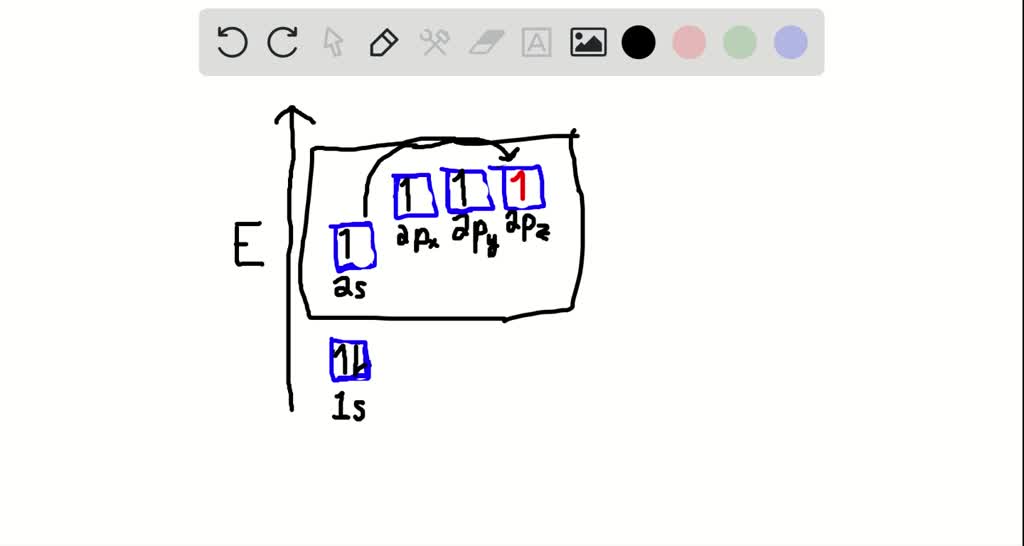
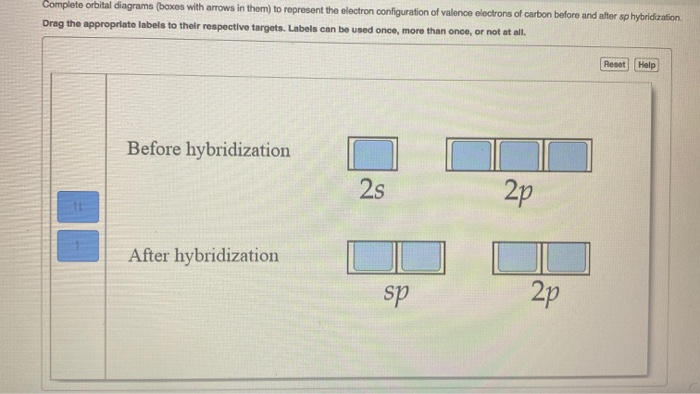
39 write the orbital diagram of carbon before sp3 hybridization
Write orbital diagrams to represent the electron configuration ... 11 Dec 2019 — Get the detailed answer: Write orbital diagrams to represent the electron configuration of carbon before sp3 hybridization. Solved Write the orbital diagram of carbon before sp ... Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 100% (6 ratings) Transcribed image text: Write the orbital diagram of carbon before sp hybridization. Use the buttons at the top of the tool to add orbitals. Click within the orbital to add electrons.
Write The Orbital Diagram Of Carbon Before Sp3 Hybridization Write the orbital diagram of carbon before sp3 hybridization. Orbital Hybridization - sp, sp 2, and sp 3 Carbon. Hybridization is used to explain molecular structures and describes the various orbital types which are involved in the bonding between atoms.
Write the orbital diagram of carbon before sp3 hybridization
NCERT Solutions for Class 11 Chemistry Chapter 11 ... - VEDANTU Ans: Carbon is sp3 hybridised in diamonds. Each carbon atom is covalently linked to four other carbon atoms. As a result of the presence of covalent connections on the surface, it has a highly rigid 3-D structure This prolonged covalent connection is extremely difficult to break, which is why diamond is the hardest material known. Write the orbital diagram to represent the electron ... Write the orbital diagram to represent the electron configuration of carbon before sp hybridization. Orbital diagram notation Condensed electron configurations are quite simple. Sp3, Sp2, And Sp Hybridization In Organic Chemistry With ... Draw the shapes of the following hybrid orbitals: sp, sp2, sp3. from ... CuBr-Catalyzed Efficient Alkynylation of sp3 C-H Bonds Adjacent to a ... sp3 Hybridization Organic Chemistry - YouTube
Write the orbital diagram of carbon before sp3 hybridization. Answered: Draw orbital diagrams (boxes with ... - bartleby 2. Draw the molecular orbital diagram of propene, CH3-CH=CH2. 3. Draw an orbital diagram of an allene, H2C=C=CH2. What hybridization must the central carbon atom have in order to form two double bonds? 4. Describe the shape of sp^3 hybrid orbitals. 5. Dicuss, with illustrations, the two main steps in the hybridization of a carbon atom. 6.a. Orbital Hybridization - Wyzant Lessons Sp 3 Hybridization. In order to understand why an orbital will engage in hybridization, we need to first look at the electron configuration. 1s 2 2s 2 2p 2 or, written another way, [He]2s 2 2p 2. The s orbital is spherical and can only hold two electrons. In contrast, we have three p orbitals which are lobed and are. OneClass: What does the atomic orbital diagram of carbon ... Write orbital diagrams to represent the electron configuration of carbon before sp3 hybridization. Solved write orbital diagrams to represent the electron ... Question: write orbital diagrams to represent the electron configuration of carbon before sp3 hybridization. This problem has been solved! See the answer ...
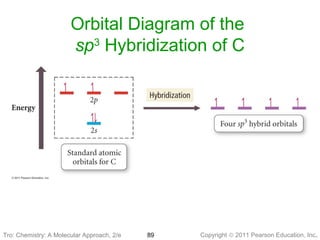
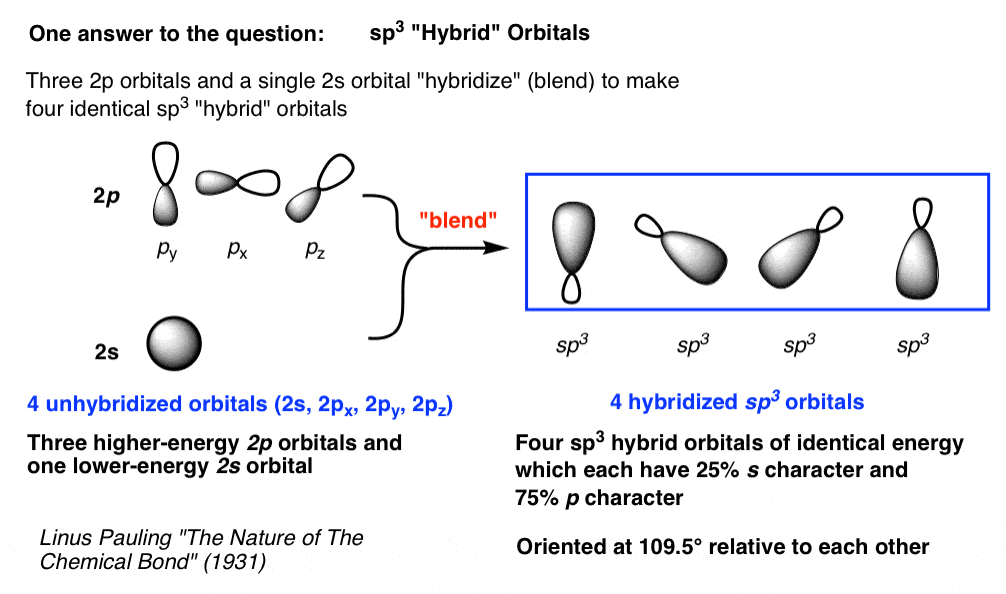
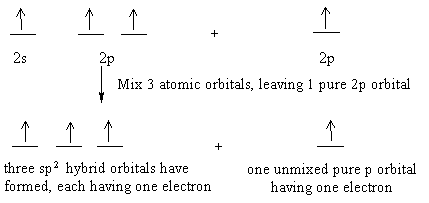
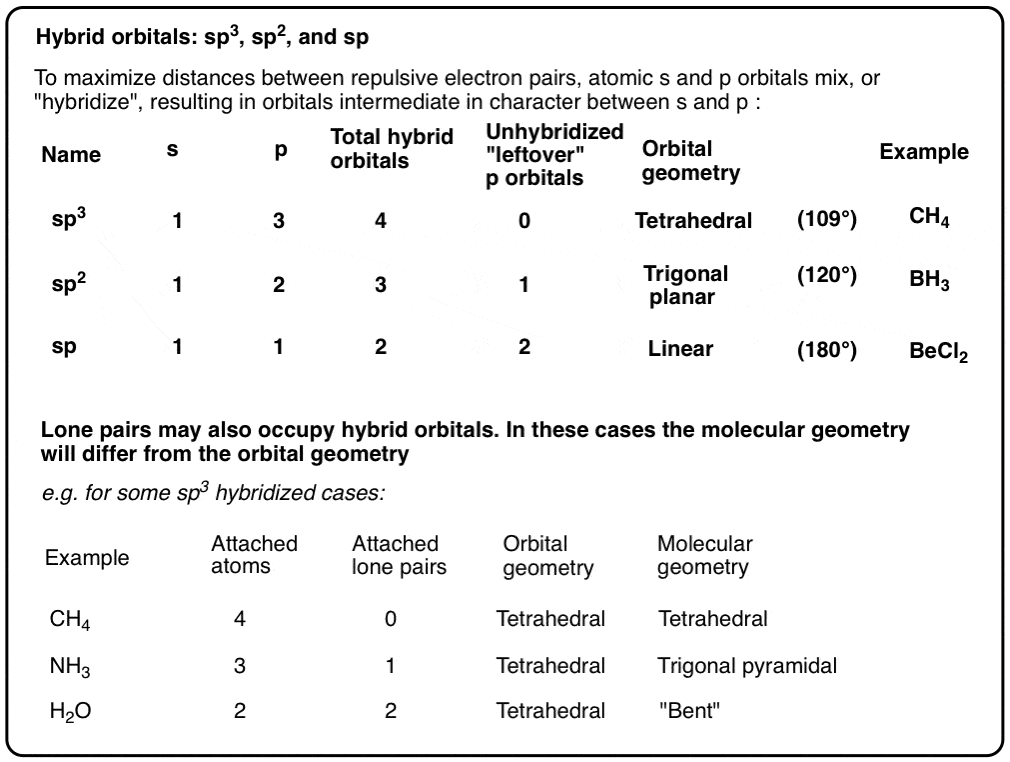
Write The Orbital Diagram Of Carbon Before Sp3 Hybridization Part L Identify the hybridization of all interior atoms for the molecule CH3 SH according. what does the atomic orbital diagram of carbon look like orbital hybridization is essentially a because the 2p orbital what does the atomic orbital diagram of carbon look like before sp 3. Hybridization of tetravalent carbon. Chem-C125 Final Exam Review Flashcards | Quizlet Assume that the energy needed for an electron in 2p orbital in an O atom to jump to 3s orbital is 3.88*10-19 J, what is its wavelength of the line atomic spectra in nanometer (nm)? 512 Given: In Atomic Spectra lab, a student obtained his best-fit line equation to be y = 0.29 x + 46.8 when he plotted his Vernier reading on the y-axis and ... Write the orbital diagram of carbon before sp3 ... Write the orbital diagram of carbon before sp 3 hybridization. Use the buttons at the top of the tool to add orbitals. Click within the orbital to add electrons. Hybridization - sp, sp2, sp3, sp3d, sp3d2 Hybridized ... The new orbitals formed are called sp 3 hybrid orbitals. These are directed towards the four corners of a regular tetrahedron and make an angle of 109°28' with one another. The angle between the sp3 hybrid orbitals is 109.28 0; Each sp 3 hybrid orbital has 25% s character and 75% p character. Example of sp 3 hybridization: ethane (C 2 H 6 ...
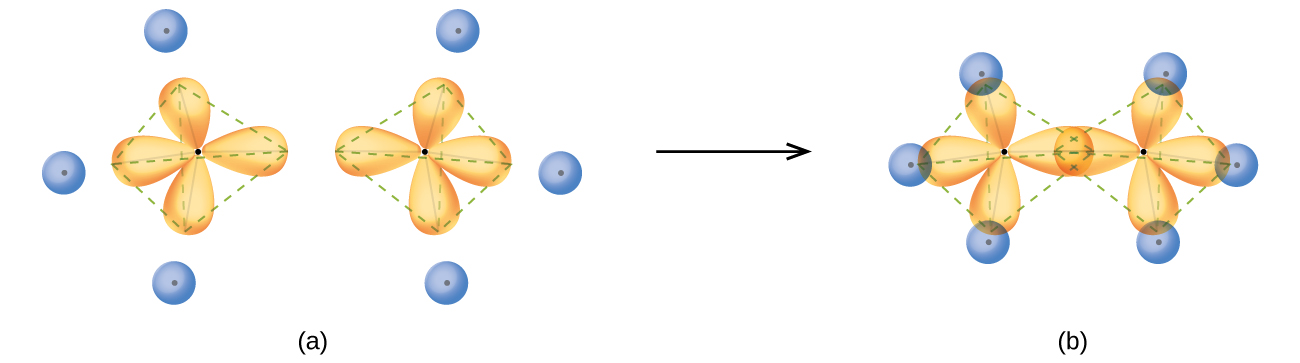
Hybridization of Atomic Orbitals - University of Illinois ... The molecular, sp 3 orbitals are arranged in a tetrahedron, with bond angles of 109.5 o. Each of the 1s orbitals of H will overlap with one of these hybrid orbitals to give the predicted tetrahedral geometry and shape of methane, CH 4. Hybridization also changes the energy levels of the orbitals. The 2s orbital of carbon is lower in energy than the 2p orbitals, since it is more penetrating. SOLVED:A nitrogen atom can undergo sp hybridization and ... A nitrogen atom can undergo sp hybridization and then become joined to carbon by a triple bond. (a) How many sigma and pi bonds make up the triple bond? (b) Draw the orbital diagram for the sp hybridization as it would look before any bonds are formed. (c) Draw sketches to show where the electron density of each bond in the triple bond is located. sp3 Hybridization | Introduction to Chemistry In hybridization, carbon's 2s and three 2p orbitals combine into four identical orbitals, now called sp 3 hybrids. The bonds between carbon and hydrogen can form the backbone of very complicated and extensive chain hydrocarbon molecules. The simplest of these is ethane (C 2 H 6 ), in which an sp 3 orbital on each of the two carbon atoms joins ... Orbital Diagram Of Carbon Before Sp3 Hybridization Write the orbital diagram of carbon before sp3 hybridization. Please just explain what the orbital looks like. So no, the atom doesn't have to get excited to 1s2 2s1 2p3 before In the case of sp3 hybridization, say in methane, the carbon s orbital. Procedure for Constructing Molecular Orbital Diagrams Based on Hybrid Orbitals.
Hybridization - Department of Chemistry Calculations done at B3LYP/6-311G+(2d,p). Click on any image above to view the optimized structure. Ethane, a two carbon molecule with a single-bond between the carbons, is the simplest alkane.. To understand the hybridization, start by thinking about the orbital diagram of the valence electrons of atomic, unhybridized carbon.
Inorganic Chemistry 4th edition, Catherine ... - Academia.edu Inorganic Chemistry 4th edition, Catherine Housecroft. 2012. Thang Pham
sp2 Hybridization | Introduction to Chemistry The p-orbitals that are unused by the carbon atoms in the hybridization overlap to form the C=C. sp 2 Hybridization in Ethene and the Formation of a Double Bond. Ethene (C 2 H 4) has a double bond between the carbons. In this case, carbon will sp 2 hybridize; in sp 2 hybridization, the 2s orbital mixes with only two of the three available 2p ...
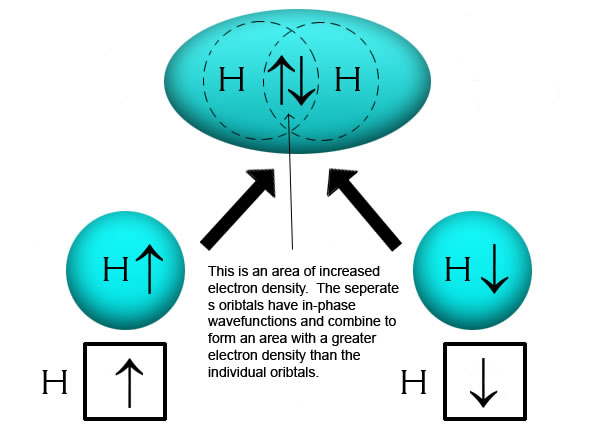
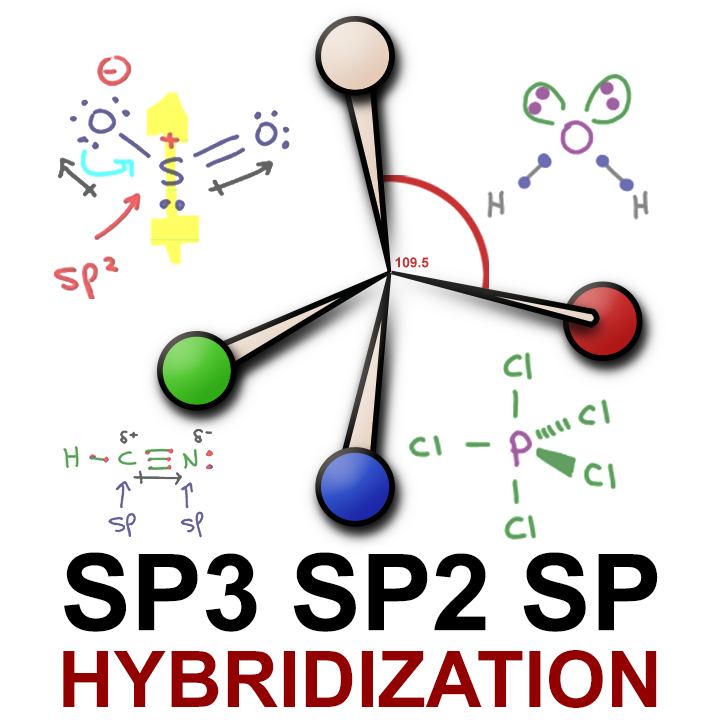
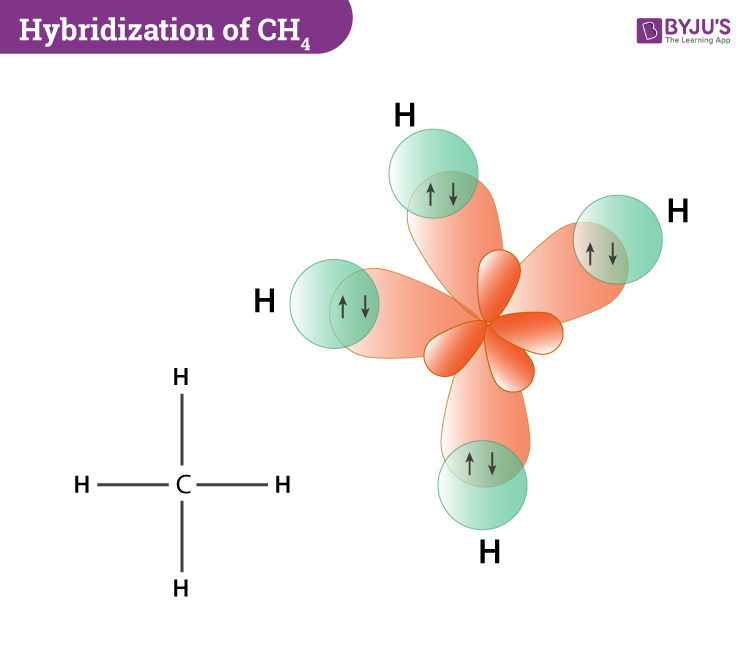
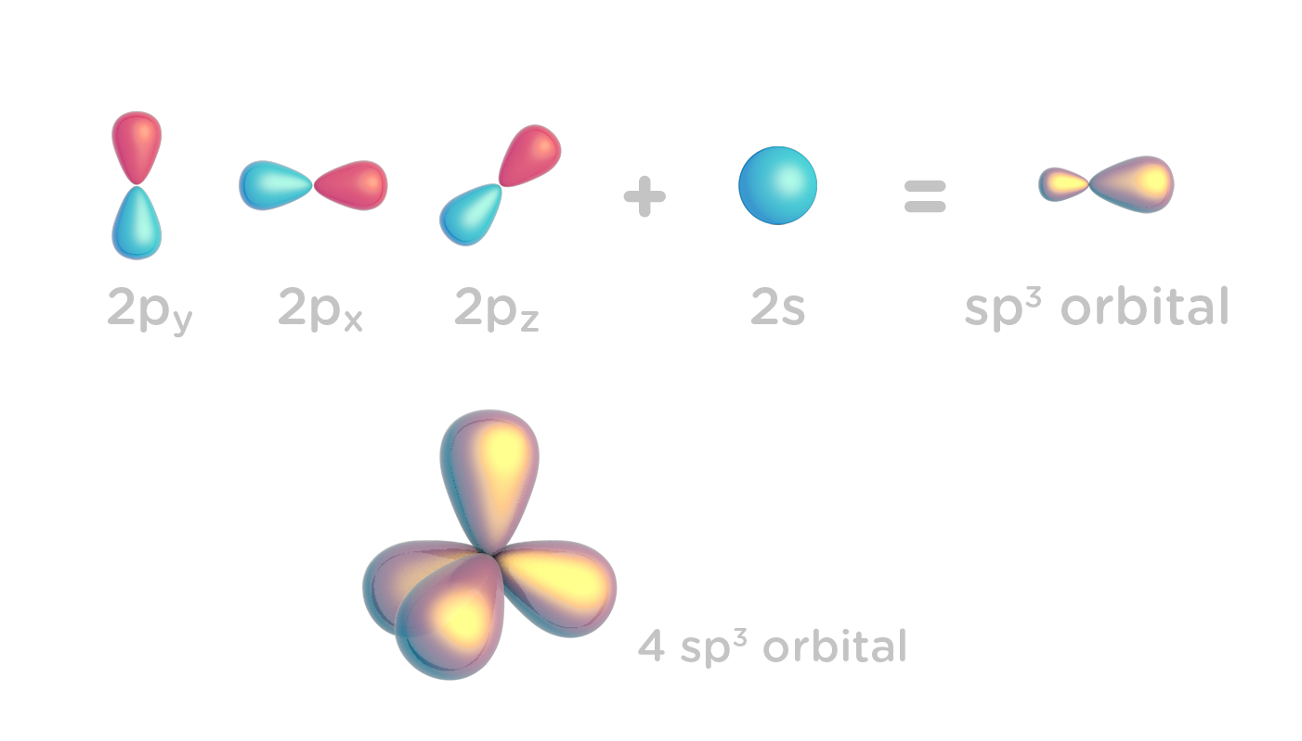
Hybridization-sp3 Hybridization-sp2 Hybridization-sp ... Methane molecule composed of one carbon atom and four hydrogen atom i.e. CH 4. In methane molecule central atom is carbon. Here carbon atom is Sp 3-hybridized. One s-orbital (2s) and three p-orbital (2px, 2py, 2pz) overlap to produce four Sp 3-hybrid orbitals. These Sp 3 - hybrid orbital are at a angle of 109.5 o from each other.
44 write the orbital diagram of carbon before sp3 ... Get the detailed answer: Write orbital diagrams to represent the electron configuration of carbon before sp3 hybridization. The compound is CH4O. The bond angles about the carbon and oxygen are 109.5 degrees because the carbon, 3 hydrogens, and oxygen form a tetrahedral shape with carbon in the center.
Write orbital diagrams (boxes with arrows in them) to ... Find step-by-step Chemistry solutions and your answer to the following textbook question: Write orbital diagrams (boxes with arrows in them) to represent the electron configurations of carbon before and after sp hybridization..
Solved Consider the electron configuration. Write ... - Chegg Consider the electron configuration. Write the orbital diagram of carbon before sp3 hybridization. Question: Consider the electron configuration. Write the orbital diagram of carbon before sp3 hybridization.
sp³ hybridization | Hybrid orbitals | Chemical bonds ... sp³ hybridization. In sp³ hybridization, one s orbital and three p orbitals hybridize to form four sp³ orbitals, each consisting of 25% s character and 75% p character. This type of hybridization is required whenever an atom is surrounded by four groups of electrons. Created by Jay. This is the currently selected item.
Opening of Epoxides With Acid - Master Organic Chemistry Feb 02, 2015 · The length of the C-O bonds will NOT be equal – the C-O bond to the tertiary carbon is longer and weaker than that of the secondary carbon. Bottom line: the tertiary carbon is more electrophilic (electron poor) and the C-O bond on the tertiary carbon is weaker, longer, and easier to break.
Hybridization of Carbon - Molecular Geometry and Bond Angles When the carbon atom is bonded to four other atoms the hybridization is said to be sp 3 type. Here 1 s orbital and 3 p orbitals in the same shell of an atom combine to form four new equivalent orbitals. The arrangement is tetrahedral with a bond angle of 109.5 o. Example: Hybridization of CH 4 (Methane) Important Points To Remember
sp3 hybridization of Carbon | CurlyArrows Chemistry Tutorials When Carbon gets a chance to form bonds with other atoms, mainly atoms of the p-block (Carbon, Hydrogen, Halogens, Oxygen, Nitrogen, etc., ) it excites one ...
Rules for Aromaticity: The 4 Key Factors – Master Organic ... Feb 23, 2017 · This lone pair can’t be in a p orbital, since the p-orbital is participating in the pi system. Instead, it’s at 90 degrees to the pi system, in the plane of the ring. In other words, the lone pair on carbon doesn’t count as a pair of pi electrons since it can’t overlap with the pi system.
Orbital Diagram Of Carbon Before Sp3 Hybridization Orbital hybridization is essentially a process of mixing orbitals together and spitting out new ones that are all identical in "symmetry" and "composition" to the orbital (s) from the other, incoming atom (s). Solution: Consider the electron configuration of a carbon schematron.org the orbital diagram of carbon before sp3 hybridization. Problem.
write orbital diagrams boxes with arrows in them to represent the electron configuration of carbon b
ekc.reitausbildung-reese.de 2 days ago · email protected] [email protected]{Praetorius2004ASG, title={A SCL4A10 gene product maps selectively to the basolateral plasma membrane of choroid plexus epithelial cells
Write orbital diagrams (boxes with arrows in them) to ... Write orbital diagrams (boxes with arrows in them) to represent the electron configurations of carbon before and after sp hybridization. - 6600924
Essentials of Physical Chemistry by B.S. Bahl ... - Academia.edu Preface The Essentials of Physical Chemistry has been written for BSc students. It has been national bestseller for more than 65 years. It has been used by more than 2 million students. It is 26 editions old. It really has been that long. A lot of
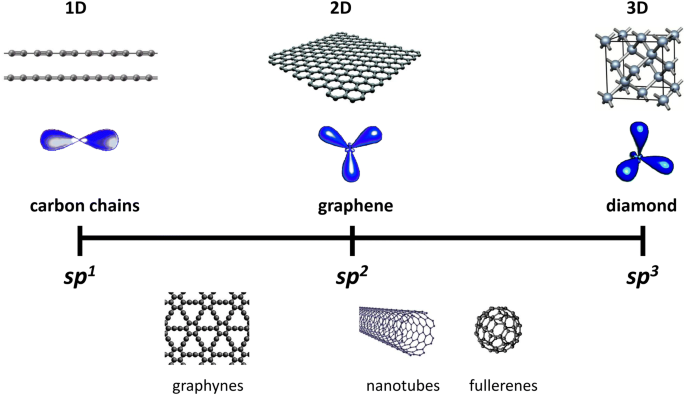
Orbital Hybridization: sp1, sp2, and sp3 Hybridization sp Hybridization: When Carbon is bound to two other atoms with the help of two double bonds or one single and one triple bond. Example: Hybridization of CO2. sp2 Hybridization: When carbon atom bonding takes place between 1 s-orbital with two p orbitals then the formation of two single bonds and one double bond between three atoms takes place. Example: Hybridization of graphite
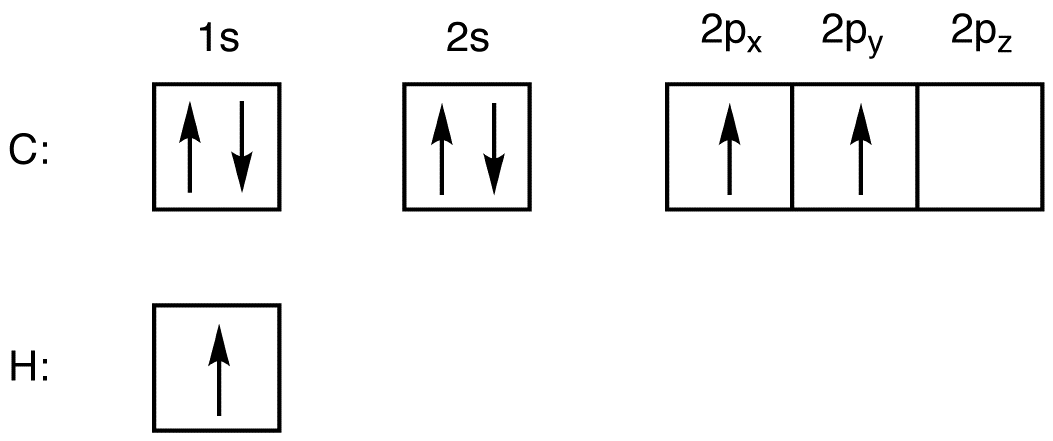
How do you write the orbital diagram for carbon? | Socratic The atomic number of carbon is 6, which is also the number of positively charged protons its atomic nuclei. If the atom is neutral, it will have the same number of negatively charged electrons. Its electron configuration is "1s"^2"2s"^2"2p"^2". The orbital diagram shows how the electrons are arranged within each sublevel. The maximum number of electrons allowed in an orbital is 2, each with ...
What is sp3 sp2 and SP carbon? - Rhumbarlv.com What is sp Hybridization. sp hybridization is the hybridization that takes place between an s atomic orbital and a p atomic orbital. An electron shell contains three p orbitals. Therefore, after the hybridization of an s orbital with one of these p orbitals, there are two un-hybridized p orbitals present in that atom .
SOLVED:Draw orbital diagrams (boxes with arrows in them ... So if we will get carbon, it has the two s and the three to pure beetles before hybridization after hybridization, it's goingto have four as p three or four. It's the way we're the Phil electrons before we hybridize is well with first and then Carbon only has a total of force. We stop here for these four orbital's.
What does the atomic orbital diagram of carbon look like ... Orbital hybridization is essentially a process of mixing orbitals together and spitting out new ones that are all identical in "symmetry" and "composition" to the orbital(s) from the other, incoming atom(s).. You can read more about #sp^3# hybridization here.The qualitative energies turn out to be the following:. with #sp^3# hybridized orbitals of #25%# #s# character and #75%# #p# character.
Sp3, Sp2, And Sp Hybridization In Organic Chemistry With ... Draw the shapes of the following hybrid orbitals: sp, sp2, sp3. from ... CuBr-Catalyzed Efficient Alkynylation of sp3 C-H Bonds Adjacent to a ... sp3 Hybridization Organic Chemistry - YouTube
Write the orbital diagram to represent the electron ... Write the orbital diagram to represent the electron configuration of carbon before sp hybridization. Orbital diagram notation Condensed electron configurations are quite simple.
NCERT Solutions for Class 11 Chemistry Chapter 11 ... - VEDANTU Ans: Carbon is sp3 hybridised in diamonds. Each carbon atom is covalently linked to four other carbon atoms. As a result of the presence of covalent connections on the surface, it has a highly rigid 3-D structure This prolonged covalent connection is extremely difficult to break, which is why diamond is the hardest material known.







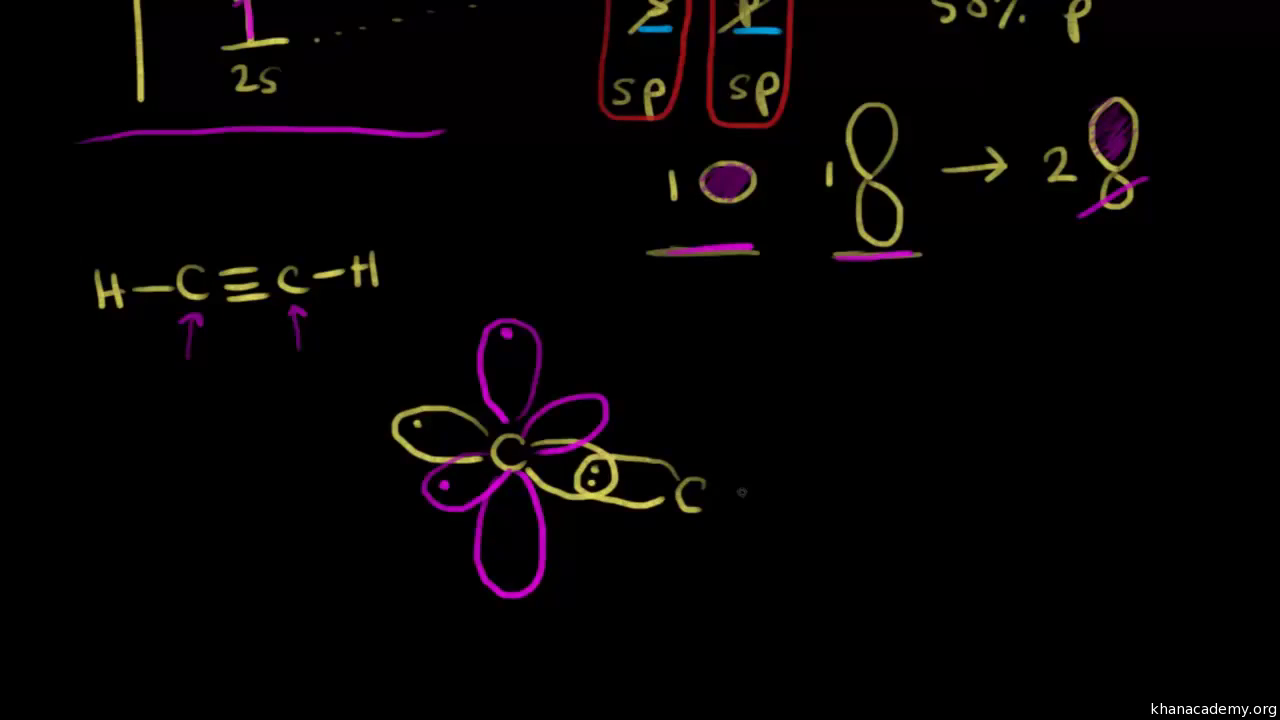






0 Response to "39 write the orbital diagram of carbon before sp3 hybridization"
Post a Comment