42 gold foil experiment diagram
Gold detector diagram - nectarhealth.com.au Gold detector diagram. The scan results are usually displayed on a computer screen or Tablet in a three-dimensional diagram. Elite Member. Metal rod has a metal knob at its top. The single-coil detector illustrated below is a simplified version of one . Adjust the stem length and coil angle so that the search coil rests flat on the ground about ... PDF Rutherford Scattering of Alphas from Thin Gold Foil and ... AN34 Experiment 15 Rutherford Scattering of Alphas from Thin Gold Foil and Other Optional Metal Foils 1 ORTEC ® Equipment Required Purpose In this experiment the scattering of alpha particles by a gold foil will be measured, and the results will be interpreted as experimental cross sections, which will be compared with theoretical equations.
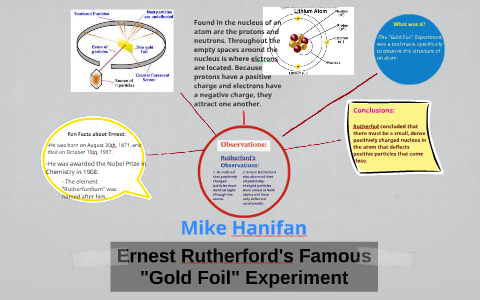
What is Gold foil experiment? - Chemistry Question - BYJUS Rutherford in 1911, carried out an experiment called 'Gold foil experiment' and could conclude the nature of an atom and the position of the protons present in the atom decisively. He also proposed the position and behaviour of electrons. They bombarded fragile sheets of gold foil with fast-moving alpha particles.

Gold foil experiment diagram
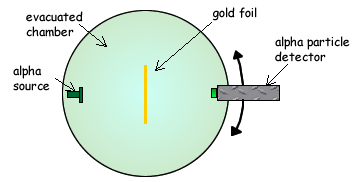
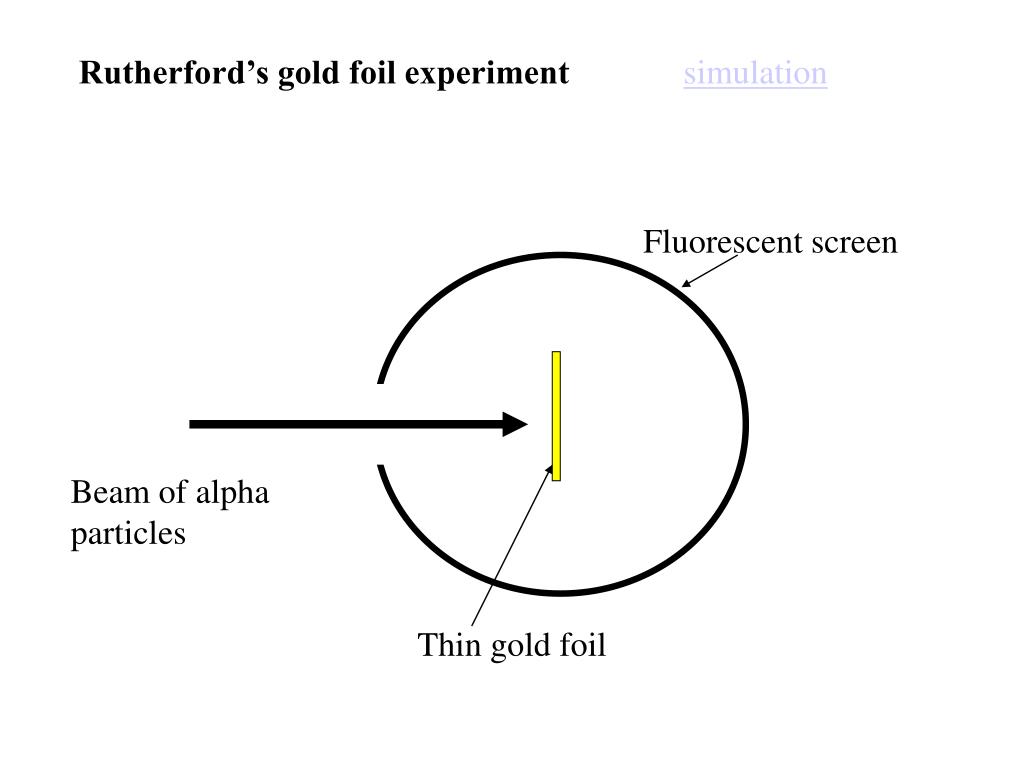
Rutherford's Gold Foil Experiment - Chemistry | Socratic Rutherford's diffraction experiment tests diffraction via a thin foil made of gold metal. Opposite the gold foil is a screen that emits a flash of light when struck by a particle. The passing of many of the particles through suggested the condensed nucleus version of the atom model. diagram of Rutherford gold foil experiment and write the ... Rutherford can use gold foil because the gold foil is very thin . and Rutherford cant explaine the stability of atom . may be help u mohsin10 mohsin10 09.12.2017 Chemistry Secondary School answered diagram of Rutherford gold foil experiment and write the observation and conclusion of his experiment 2 Ernest Rutherford's Gold Foil Experiment: Physics Lab ... Ernest Rutherford's gold foil experiment helped scientists understand the charge of an atom. Follow along with Rutherford's experiment in this lab and understand the surprising results and ...
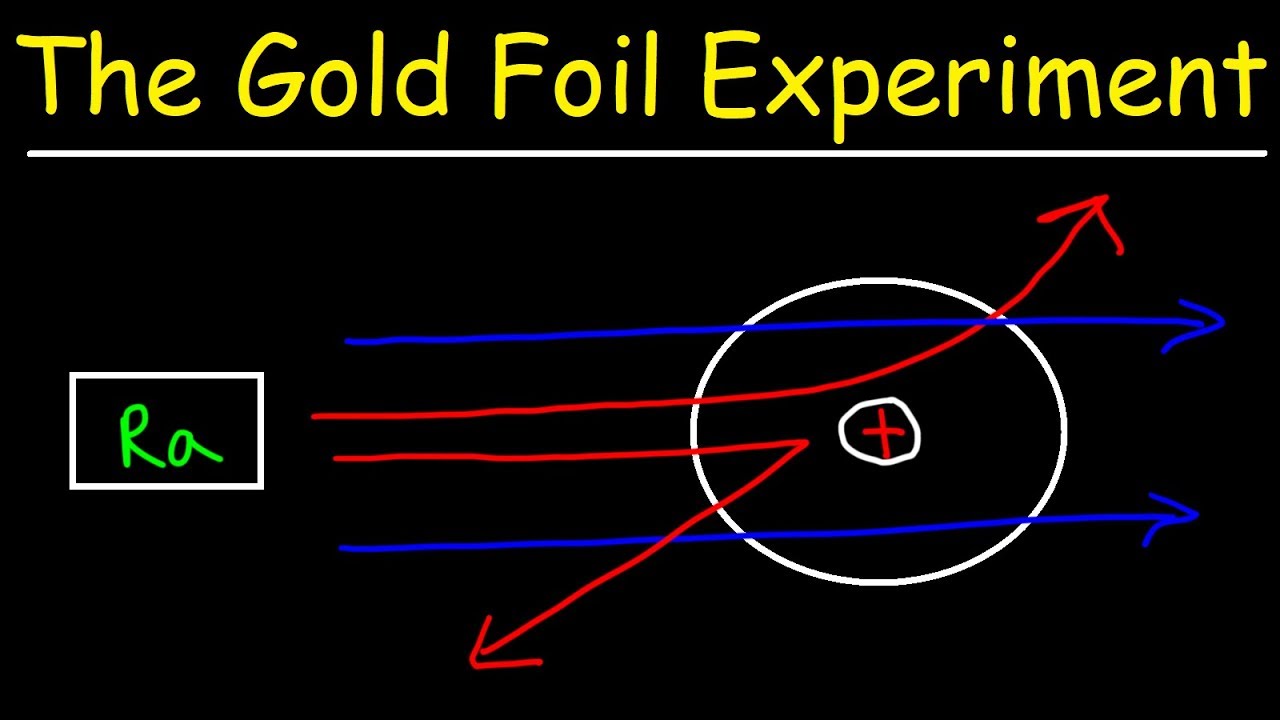
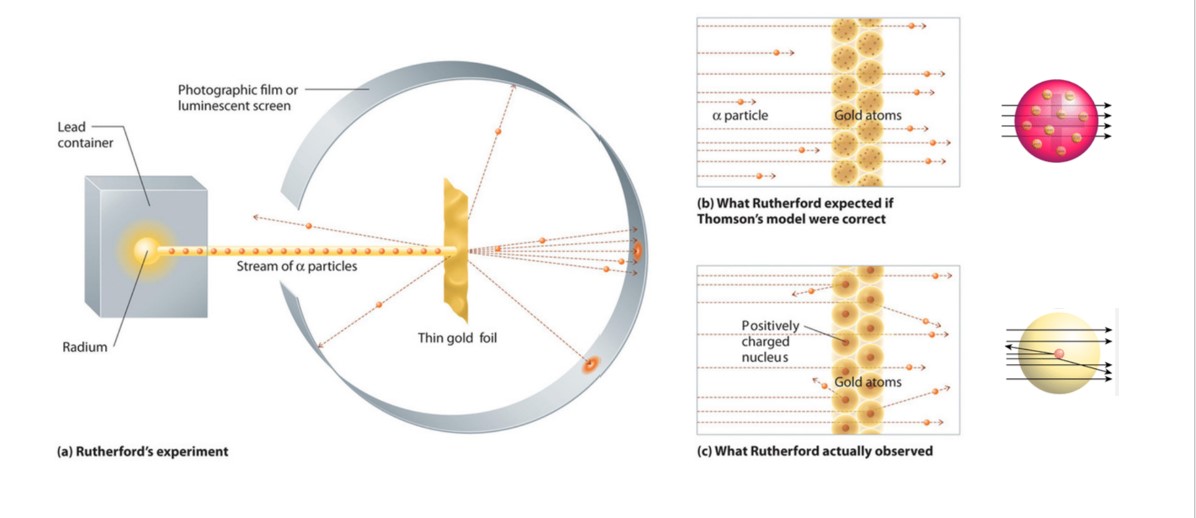
Gold foil experiment diagram. Gold Foil Experiment | Ernest Rutherford & Results - Video ... The gold foil experiment results in the Rutherford model, where the atom is composed of a positively charged nucleus surrounded by negatively charged electrons. The Ernest Rutherford model of the... Rutherford Model of the Atom: Definition & Diagram - Video ... This diagram depicts the expected and the actual results of the gold foil experiment. The diagram on the left shows particles passing through the positively charged matrix of the plum pudding... Explain the gold foil experiment by Rutherford. What were ... Rutherford's Gold Foil Experiment: Rutherford took a very thin gold foil with 100 nm thickness. He bombarded the gold foil with alpha rays. It is important to note that alpha particles are positively charged. Rutherford's gold foil experiment (video) - Khan Academy Rutherford's gold foil experiment. This is the currently selected item. Bohr's model of hydrogen. Next lesson. Bohr's model of the hydrogen atom. Video transcript - [Voiceover] This is a quote by a physicist as a comment on one of his experimental results. He said, about his experiment, he said, "It was as if you fired a 15-inch shell "at a ...
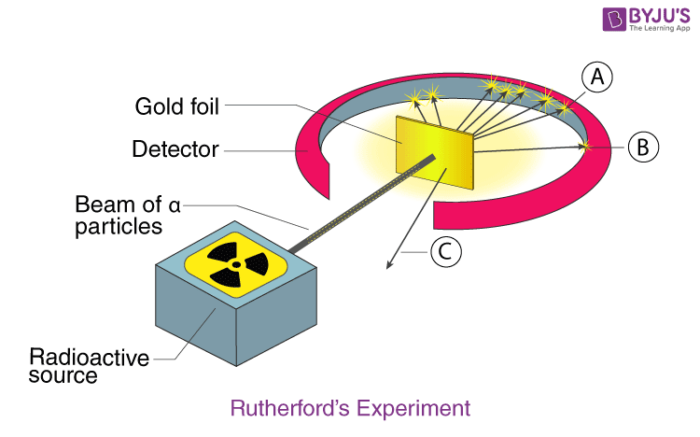
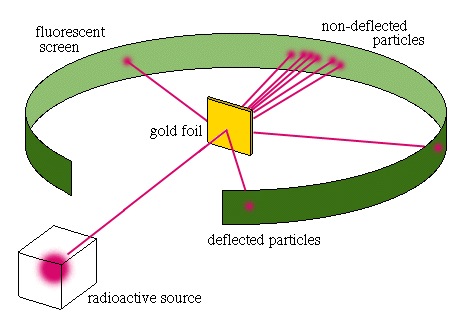
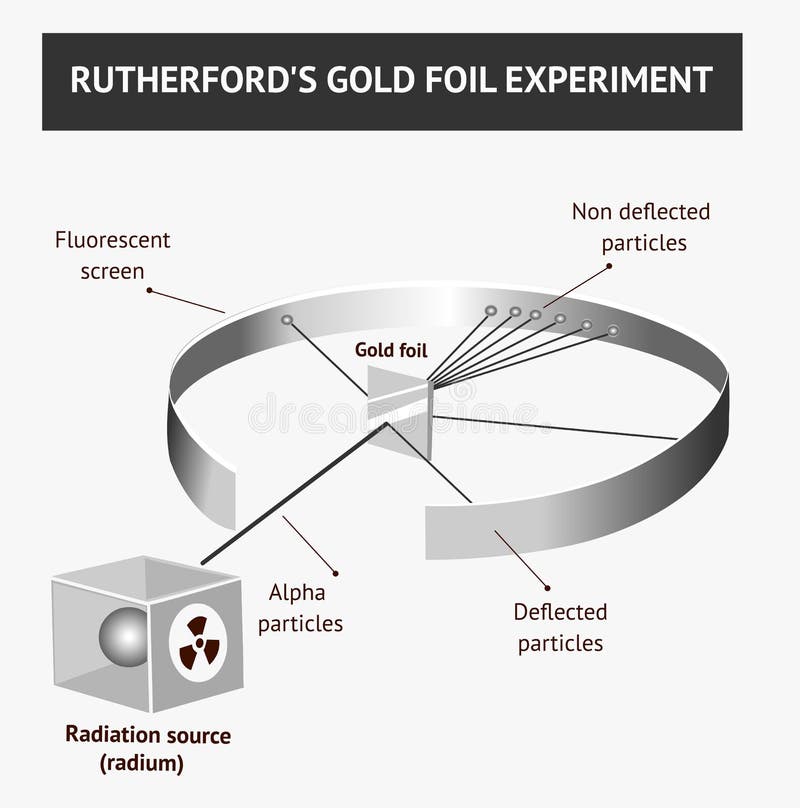
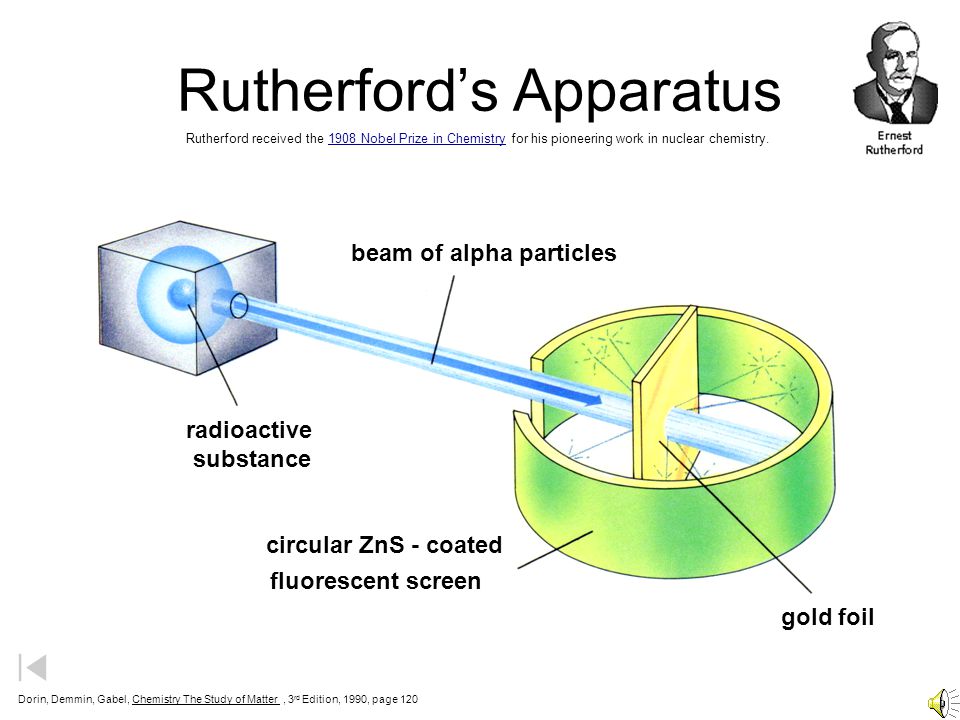
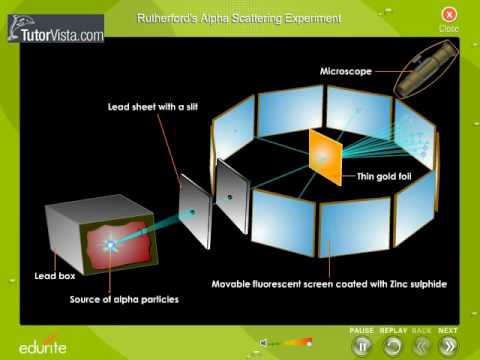
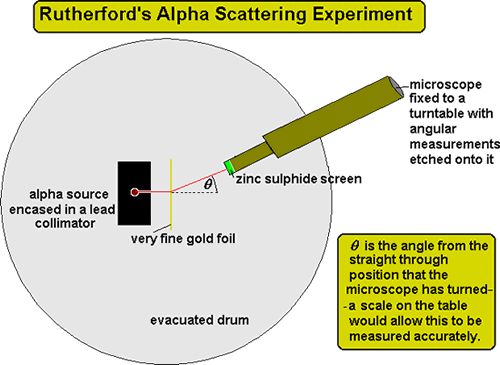
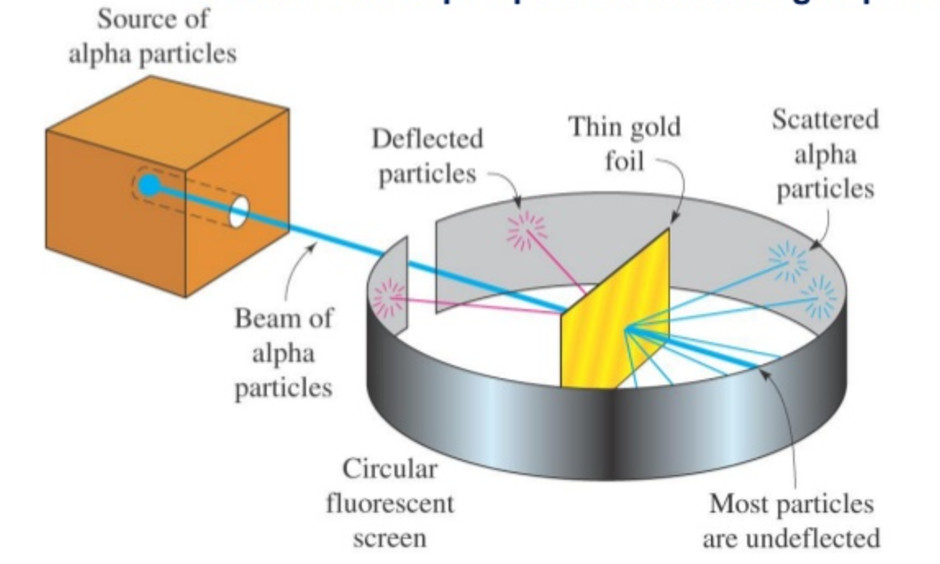
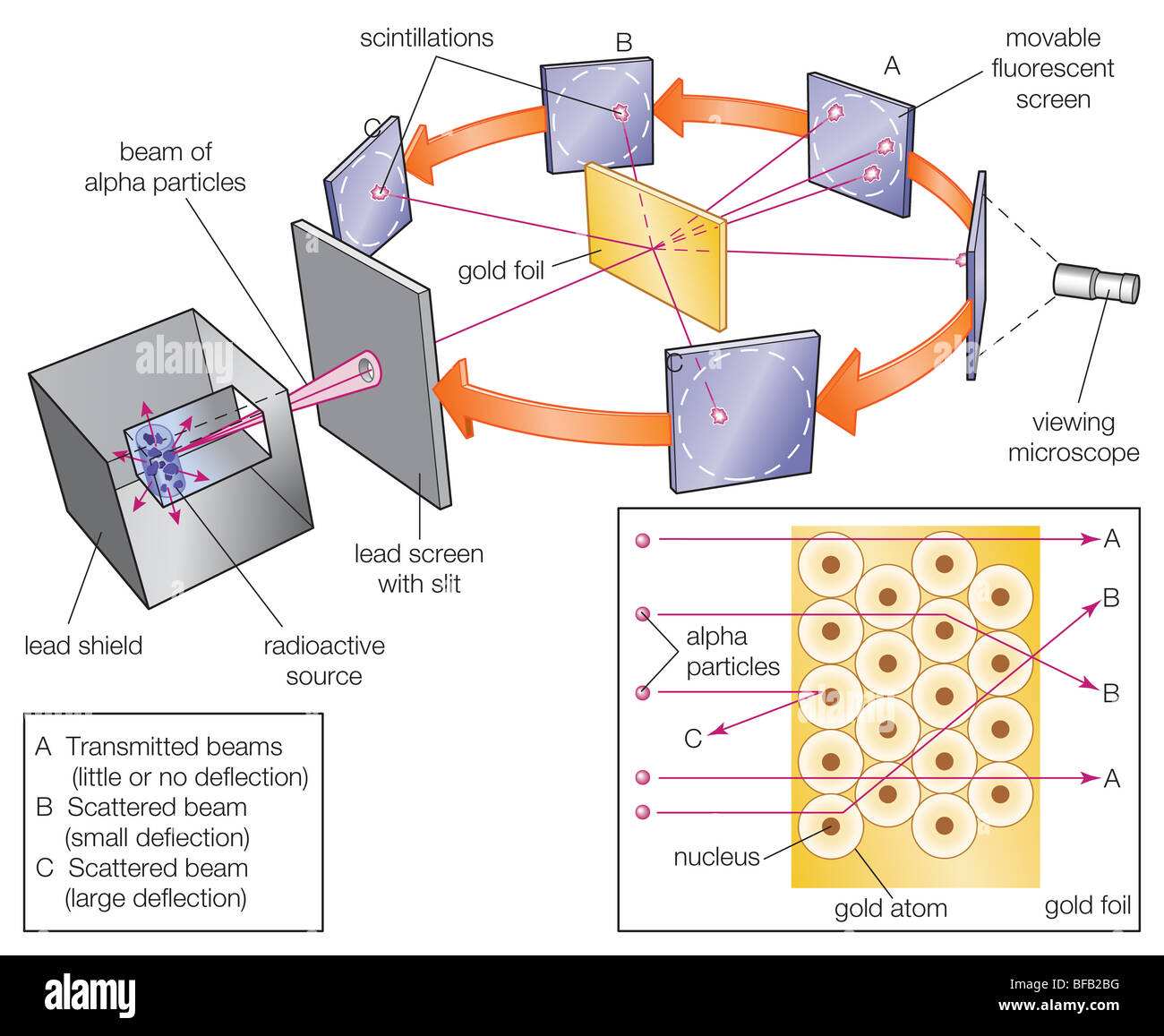
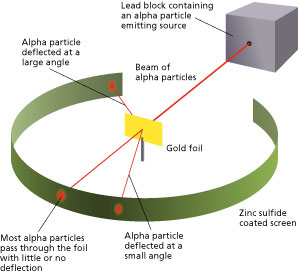
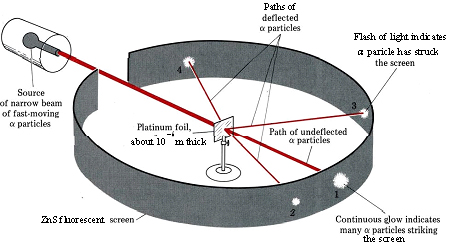
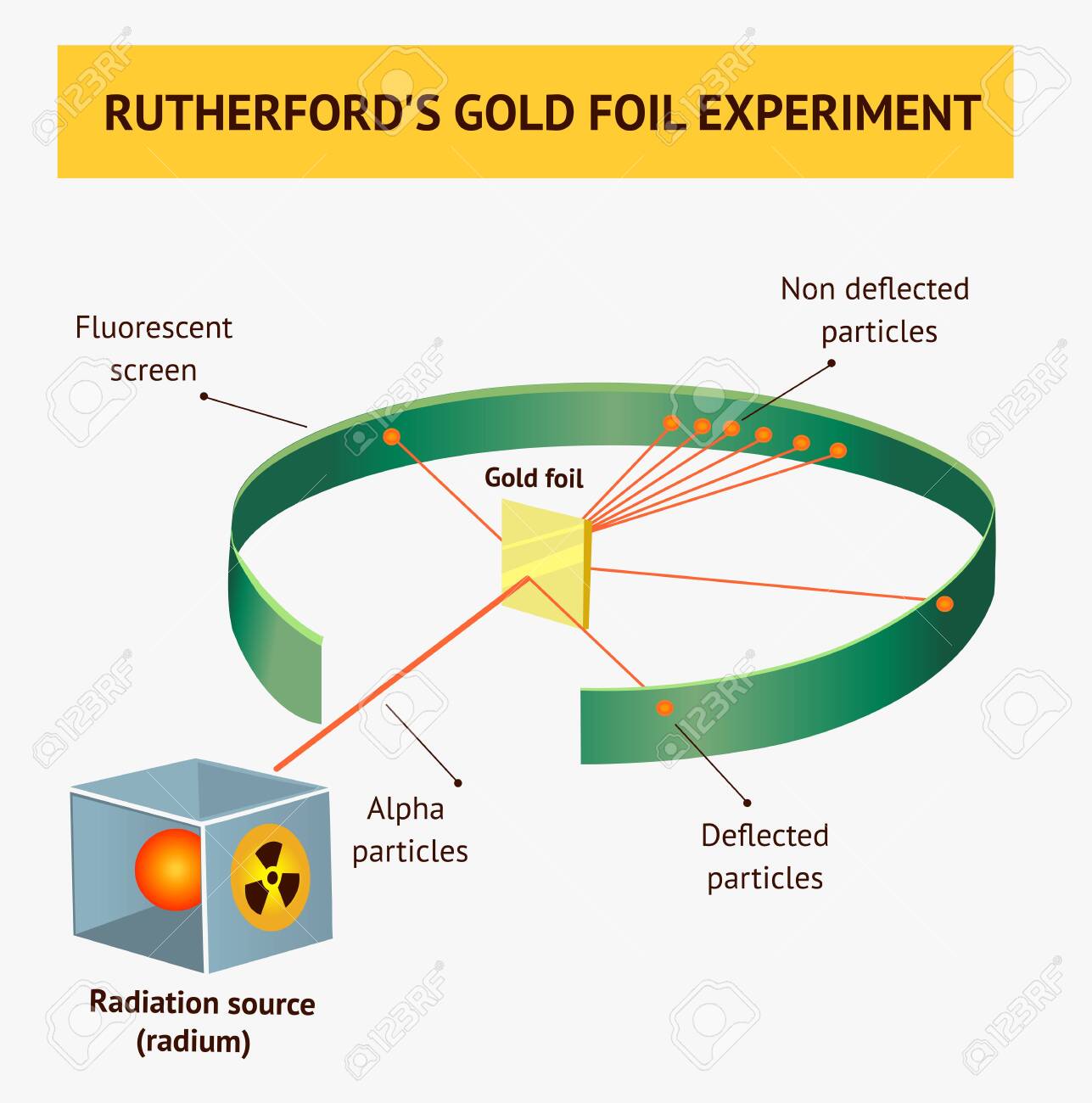
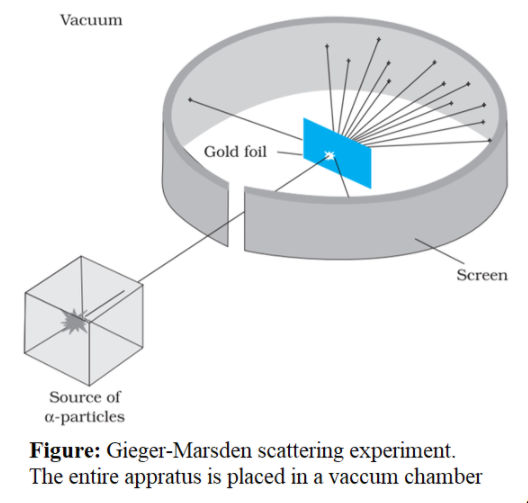
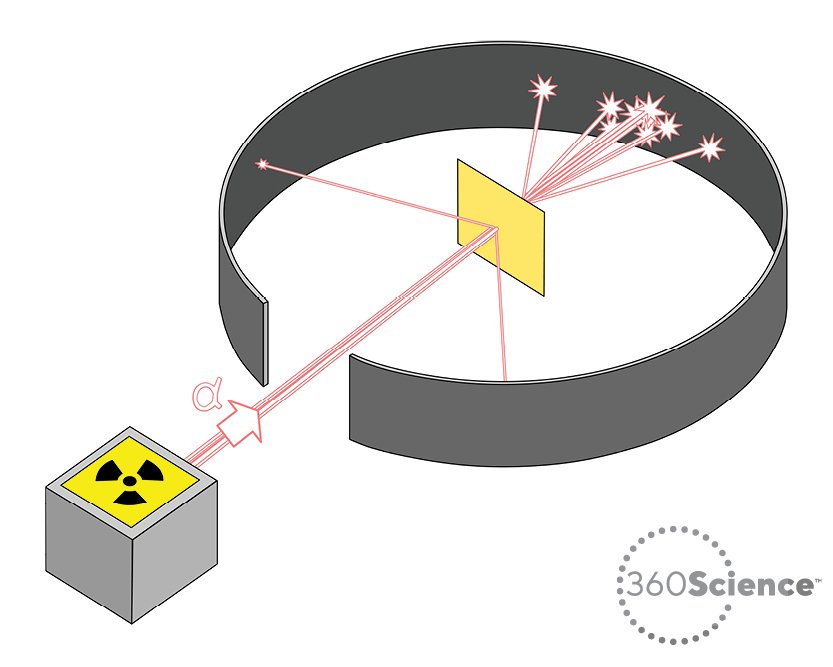
Gold Foil Experiment- Video quiz. Diagram | Quizlet Start studying Gold Foil Experiment- Video quiz.. Learn vocabulary, terms, and more with flashcards, games, and other study tools. how to draw rutherford scattering experiment| how to draw ... Title: how to draw rutherford scattering experiment| how to draw diagram of rutherford gold foil experiment | rutherford alpha particle scattering experiment... Explain Rutherford's alpha - ray scattering experiment ... Rutherford passed beams of alpha particles through a thin gold foil and noted how the alpha particles scattered from the foil.. Observations of Rutherford's alpha ray scattering experiment:. 1. Most of the α-particles passed straight through the gold foil without any deviation.. 2. Some of the α-particles were deflected by the foil by some angles. Rutherford's Experiments (M2Q2) - UW-Madison Chemistry 103 ... Geiger and Rutherford fired α particles at a piece of gold foil and detected where those particles went, as shown in this schematic diagram of their experiment. Most of the particles passed straight through the foil, but a few were deflected slightly and a very small number were significantly deflected.
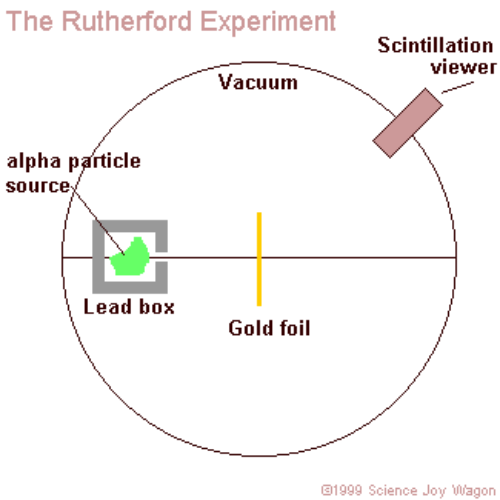
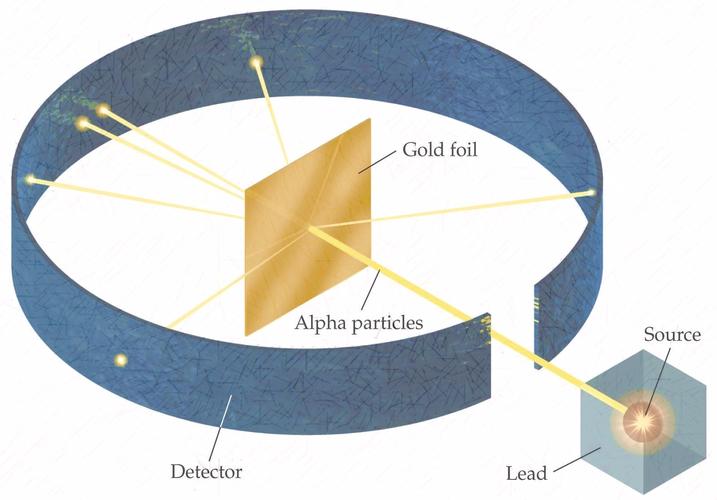
M2Q2: Rutherford's Experiments - Chem 103/104 Resource Book Geiger and Rutherford fired α particles at a piece of gold foil and detected where those particles went, as shown in this schematic diagram of their experiment. Most of the particles passed straight through the foil, but a few were deflected slightly and a very small number were significantly deflected. Rutherford's Alpha Scattering Experiment - GeeksforGeeks Rutherford's Alpha Scattering Experiment. set-up of Rutherford's experiment. He conduct an experiment by bombarding alpha particles into a thin sheet of gold and then notices their interaction with the gold foil and trajectory or path followed by these particles. In the experiment, Rutherford passes very high streams of alpha-particles from ... Rutherford's Gold Foil Experiment - ChemistryGod Rutherford's gold foil experiment When Rutherford along with his colleague shot alpha particles, the positively charged helium nuclei, on a very thin gold foil, unexpected scattering of the particles was observed. The illustration above depicts a radioactive source enclosed in a lead block liberates alpha particles. Rutherford and the nucleus - BBC Bitesize In 1905, Ernest Rutherford did an experiment to test the plum pudding model. His two students, Hans Geiger and Ernest Marsden, directed a beam of alpha particles at a very thin gold leaf suspended ...
PDF The Rutherford Scattering Experiment alpha beam, and this can be covered with gold foil to fully simulate the energy loss in the gold foil scattering experiment. 3. Figure 4: This image shows the cage with alpha source and gold scattering foil prepared for a ... The block diagram of the front end of the system (up to the pulse height analysis) is shown in Figure 5.
Geiger-Marsden experiments - Wikipedia The Geiger-Marsden experiments (also called the Rutherford gold foil experiment) were a landmark series of experiments by which scientists learned that every atom has a nucleus where all of its positive charge and most of its mass is concentrated. They deduced this after measuring how an alpha particle beam is scattered when it strikes a thin metal foil.
Rutherford's Scattering Experiment (Theory) : Class 9 ... Rutherford designed an experiment for this. In this experiment, fast moving alpha (α)-particles were made to fall on a thin gold foil. He selected a gold foil because he wanted as thin a layer as possible. This gold foil was about 1000 atoms thick. α-particles are doubly-charged helium ions.
Ernest Rutherford's Gold Foil Experiment: Physics Lab ... Ernest Rutherford's gold foil experiment helped scientists understand the charge of an atom. Follow along with Rutherford's experiment in this lab and understand the surprising results and ...
diagram of Rutherford gold foil experiment and write the ... Rutherford can use gold foil because the gold foil is very thin . and Rutherford cant explaine the stability of atom . may be help u mohsin10 mohsin10 09.12.2017 Chemistry Secondary School answered diagram of Rutherford gold foil experiment and write the observation and conclusion of his experiment 2
Rutherford's Gold Foil Experiment - Chemistry | Socratic Rutherford's diffraction experiment tests diffraction via a thin foil made of gold metal. Opposite the gold foil is a screen that emits a flash of light when struck by a particle. The passing of many of the particles through suggested the condensed nucleus version of the atom model.

















0 Response to "42 gold foil experiment diagram"
Post a Comment