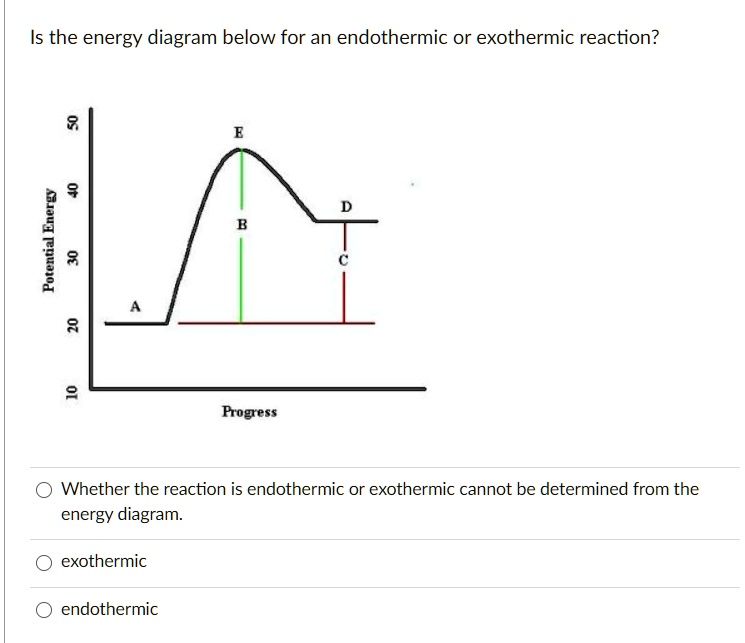
42 potential energy diagram exothermic
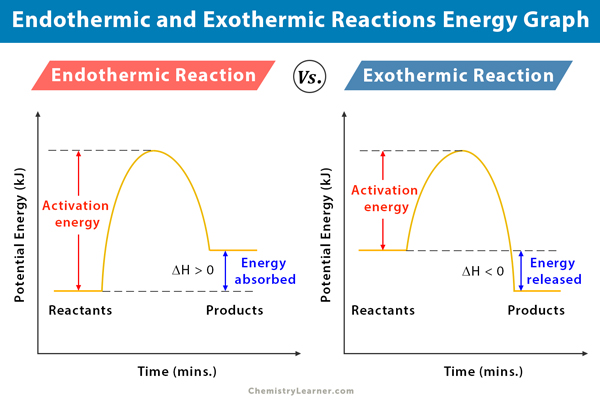
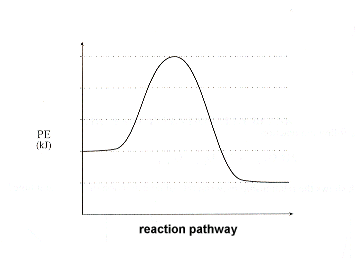
How can I represent an exothermic reaction in a potential ... Chemistry Chemical Kinetics Potential Energy Diagrams 1 Answer Stefan V. Dec 19, 2014 An exothermic reaction is a reaction in which energy is given off, or, in other words, a reaction that has a ΔH < 0 (see: enthalpy ). Here's a potential energy diagram for an exothermic reaction, the combustion of glucose Energy Diagrams of Reactions | Fiveable In the graph for an exothermic reaction, you can see that the products have a lower potential energy, implying that energy has been released from the system. This further proves that ΔH is negative in an exothermic reaction. Image Courtesy of SilaVula Endothermic Reactions
Classify each chemical reaction as exothermic or ... Classify each chemical reaction as exothermic or endothermic. Potential energy diagram for the reaction Potential energy diagram for the reaction 24,0 + 572 kJ → 2H2 + O2 H₂.0₂ Potential Energy H,O Time o exothermic O endothermic DONE
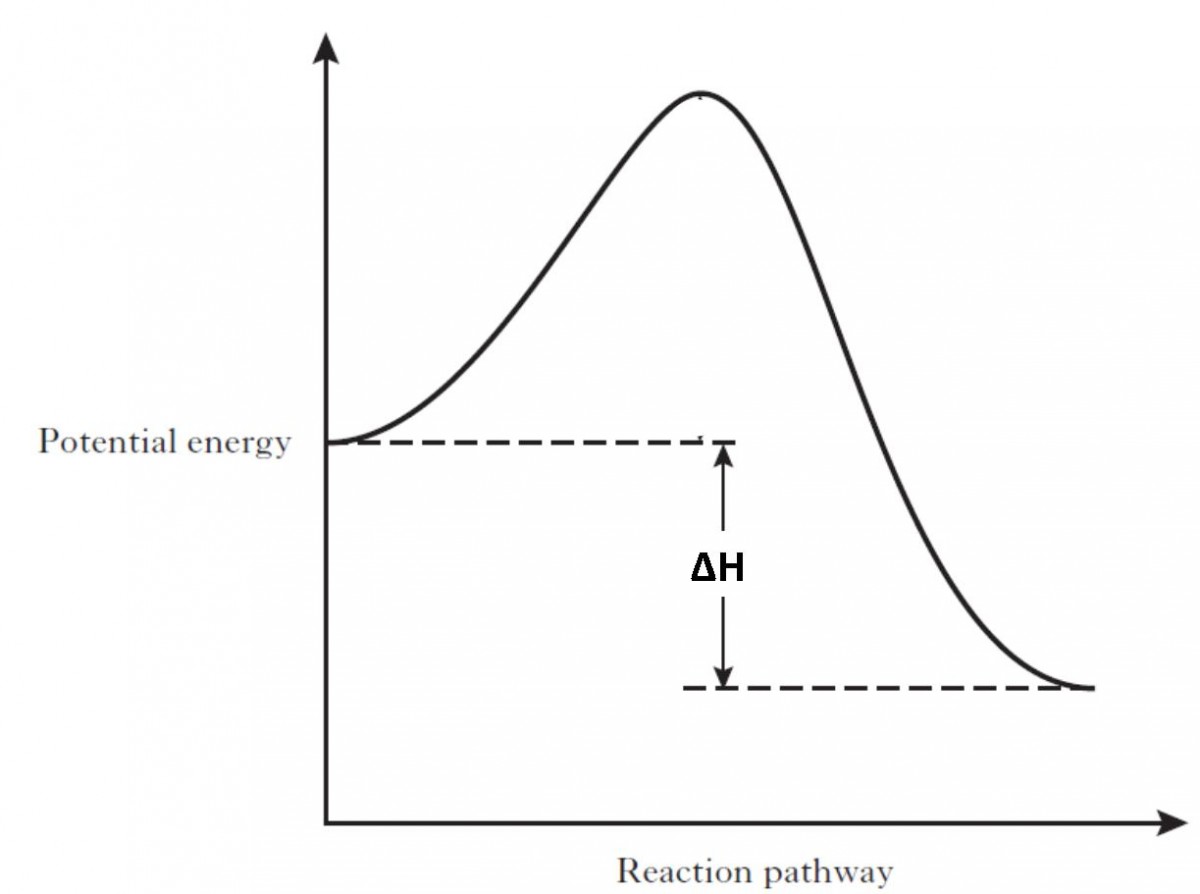
Potential energy diagram exothermic
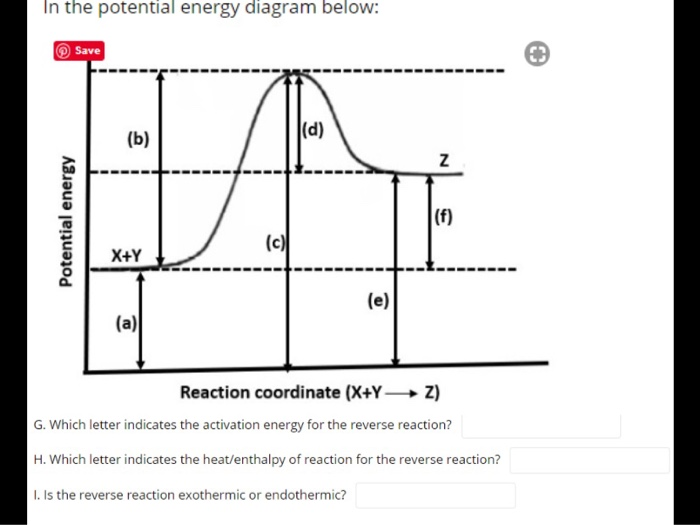
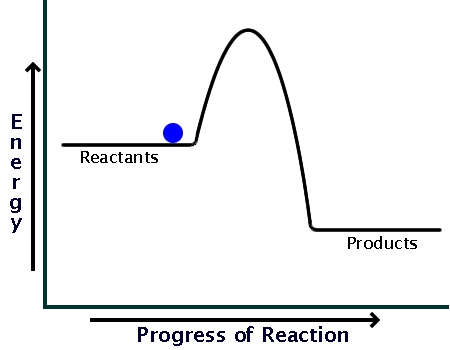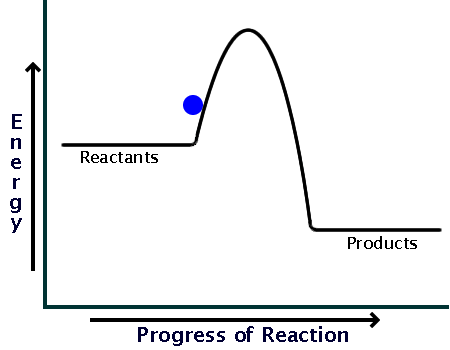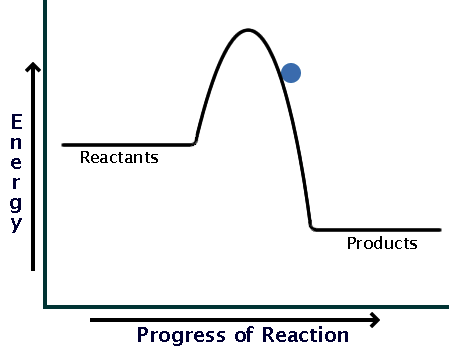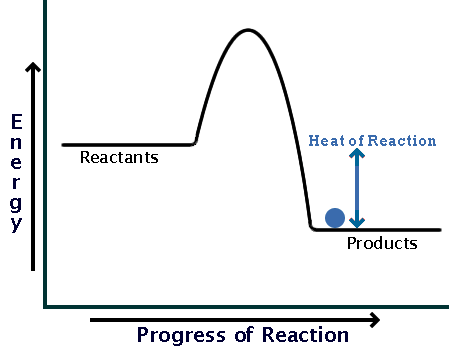
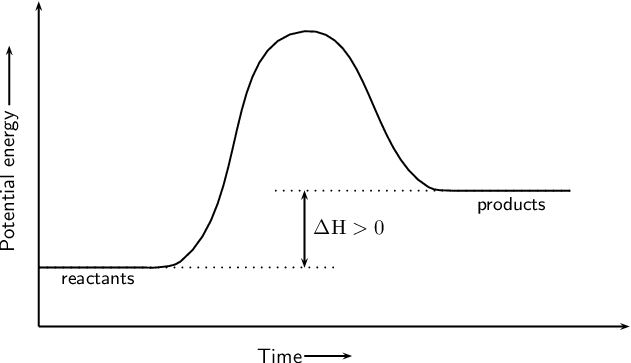
estebantorreshighschool.com › equation-help › how-toHow to tell if a reaction is exothermic or endothermic from ... Image of a graph showing potential energy in relation to the process of a chemical reaction. In the case of an exothermic reaction, the reactants are at a higher energy level as compared to the products, as shown below in the energy diagram. In other words, the products are more stable than the reactants. socratic.org › questions › 577fcfdc11ef6b60ad515f59What is the activation energy for the reverse reaction in ... Aug 03, 2016 · This is asking you to draw a potential energy diagram for an endothermic reaction. Recall that DeltaH_"rxn", the enthalpy of reaction, is positive for endothermic reactions, i.e. the product(s) (right) are higher in energy than the reactant(s) (left) and energy was absorbed. (Energy increases from bottom to top.) Since... the activation energy for the forward reaction is the difference in ... Heat Of Reaction Amd Potential Energy Diagram potential energy of the reactants is equal to the (1) heat of fusion (2) heat of reaction (3) activation energy of the forward reaction (4) activation energy of the reverse reaction 5 The energy absorbed and the energy released during a chemical reaction are best represented by a (1) cooling curve (2) heating curve (3) kinetic energy diagram
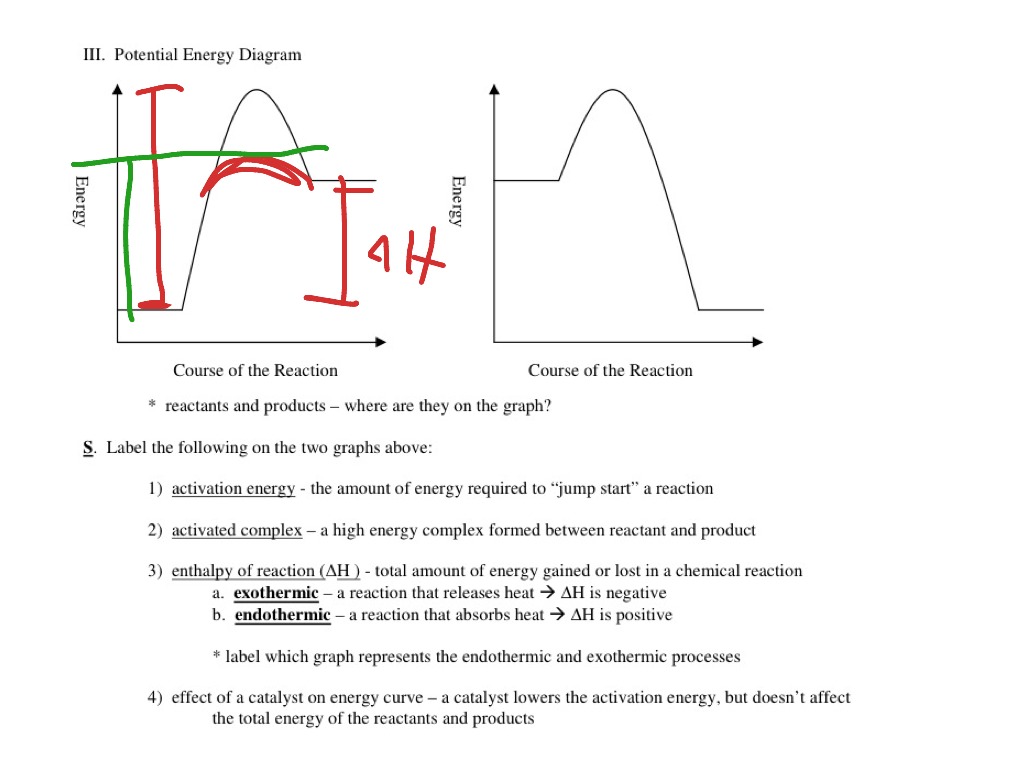
Potential energy diagram exothermic. › series › chemistry-challenges-andThe Energy in Chemical Reactions: Thermodynamics and Enthalpy ... Objects can also possess potential energy. An object has potential energy because of its position in relation to other objects. When an object rises against the force of gravity, it gains potential energy; when it falls, it loses potential energy. The object stores this energy as it rises and releases it as it returns to Earth. Potential Energy Diagrams - Chemistry - Catalyst ... This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f... Potential Energy Diagrams - Kentchemistry.com A potential energy diagram plots the change in potential energy that occurs during a chemical reaction. This first video takes you through all the basic parts of the PE diagram. Sometimes a teacher finds it necessary to ask questions about PE diagrams that involve actual Potential Energy values. PDF Potential Energy Diagrams THE DIAGRAMS GRAPHICALLY COMPARE REACTANT ENERGY TO PRODUCT ENERGY AS REACTION OCCURS IF REACTANTS HAVE MORE ENERGY THEN PRODUCTS, THE REACTION IS EXOTHERMIC (loss of energy) IF REACTANTS HAVE LESS ENERGY THEN PRODUCTS, THE REACTION IS ENDOTHERMIC (gain of energy) ENDOTHERMIC OR EXOTHERMIC? Note the REACTANTS and PRODUCTS are labeled.
What statement describes the potential energy diagram of ... What statement describes the potential energy diagram of an exothermic reaction? The potential energy of the reactants is greater than the potential energy of the products. How does the energy level diagram show this reaction is ... Figure shows the energy level diagram for the reaction between methane and oxygen. Based on Figure, the following information can be obtained. (a) The reaction between methane and oxygen to form carbon dioxide and water is an exothermic reaction. (b) During the reaction, the temperature of the mixture increases. Endothermic and Exothermic Reactions With Potential Energy ... This chemistry video tutorial provides a basic introduction into endothermic and exothermic reactions as well as the corresponding potential energy diagrams.... Potential energy diagrams - Controlling the rate - Higher ... An exothermic reaction is one in which heat energy is given out. The products must have less energy than the reactants because energy has been released. This can be shown by a potential energy...
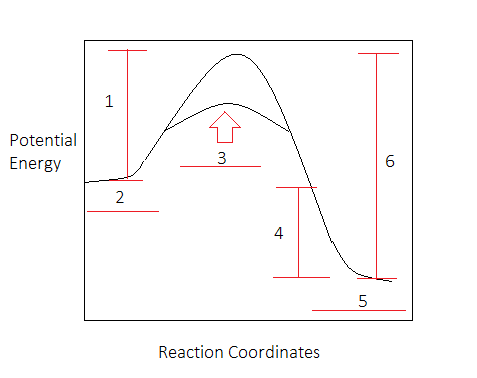
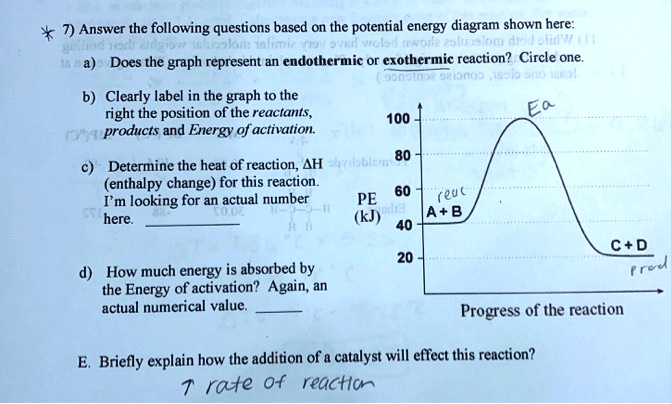
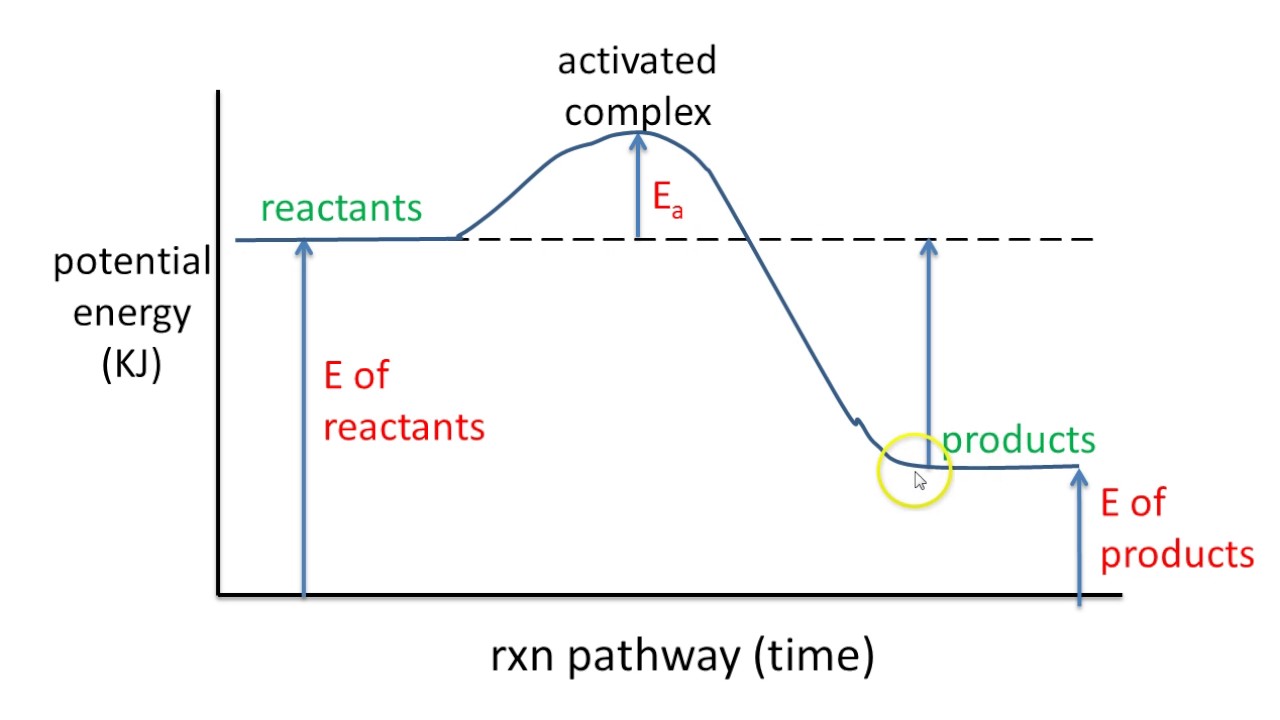
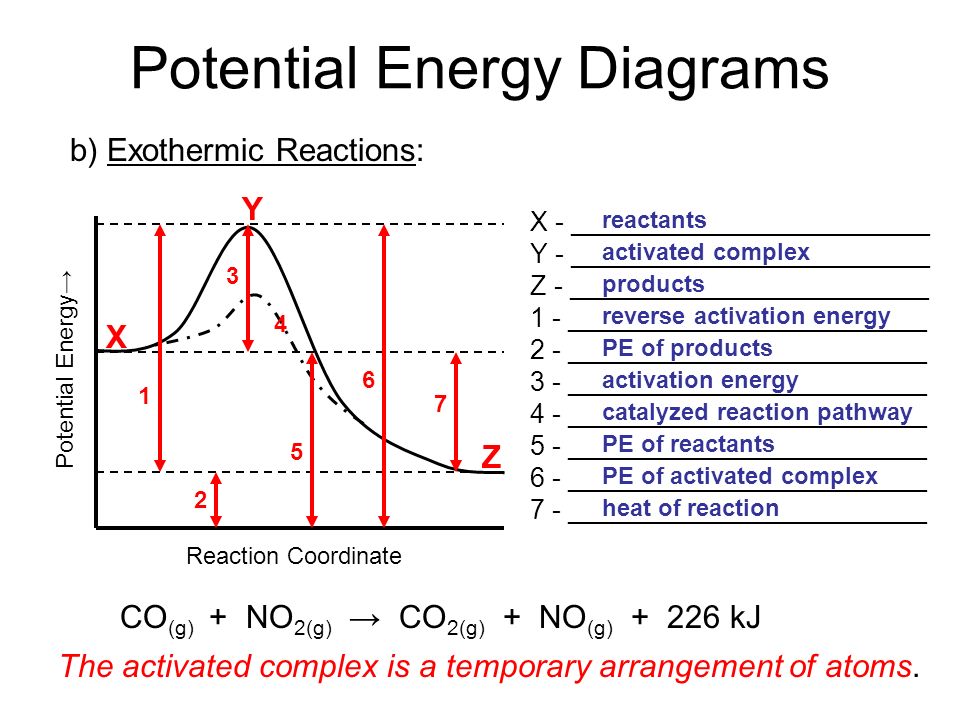
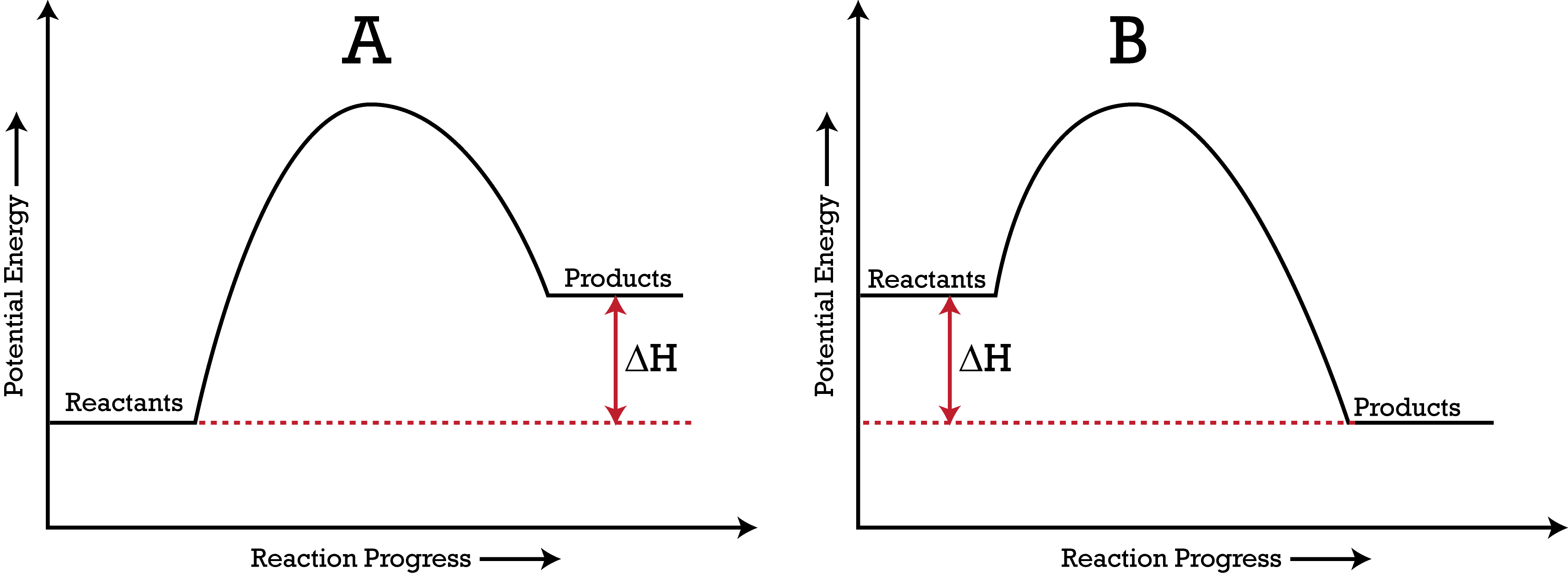
18.4: Potential Energy Diagrams - Chemistry LibreTexts A potential energy diagram shows the change in potential energy of a system as reactants are converted into products. The figure below shows basic potential energy diagrams for an endothermic (A) and an exothermic (B) reaction. Recall that the enthalpy change ( Δ H) is positive for an endothermic reaction and negative for an exothermic reaction. Transition State Potential Energy Diagram - surface ... We undertake this nice of Transition State Potential Energy Diagram graphic could possibly be the most trending topic in the manner of we allowance it in google gain or facebook. We attempt to introduced in this posting past this may be one of astounding mention for any Transition State Potential Energy Diagram options. Answered: Consider the potential energy diagram… | bartleby Consider the potential energy diagram shown below. This graph shows the chemical potential energy in a reaction system over time. The y-axis is potential energy in kilojoules. The x-axis is the reaction progress, or time. Does this graph represent an endothermic or an exothermic reaction? Explain your answer. PDF NAME Kinetics Potential Energy Diagrams - ISD 622 Potential energy diagrams are used to represent potential energy changes that take place as time progresses during chemical reactions. The potential energy diagrams for the exothermic reaction between substances X and Y is shown below. The reaction progresses from stage 1 (reactants), through stage 2 (activated complex to), to stage 3 (products ...
Solved Select the potential energy diagram that represents ... Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Exothermic reactions are characterized by negative change in …. View the full answer. Transcribed image text: Select the potential energy diagram that represents an exothermic (exergonic) reaction.
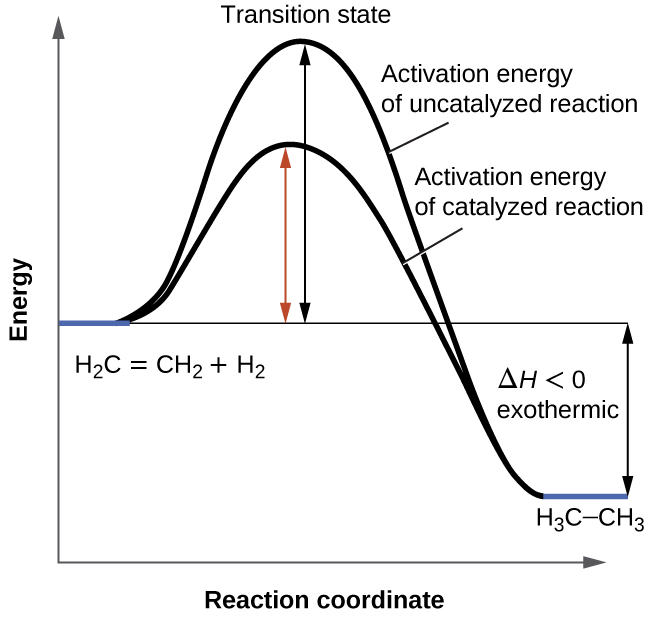
Representing endothermic and exothermic processes using ... The peaks in energy diagrams for both endothermic and exothermic reaction energy diagrams are known as the transition state or the activation complex. In a reaction, any reaction, the same general trend occurs. First the bonds of the reactants are broken which requires an input of energy to be put into the reaction.
phet.colorado.edu › en › simulationsReactions & Rates - Reaction | Kinematics | Concentration ... Explore what makes a reaction happen by colliding atoms and molecules. Design experiments with different reactions, concentrations, and temperatures. When are reactions reversible?
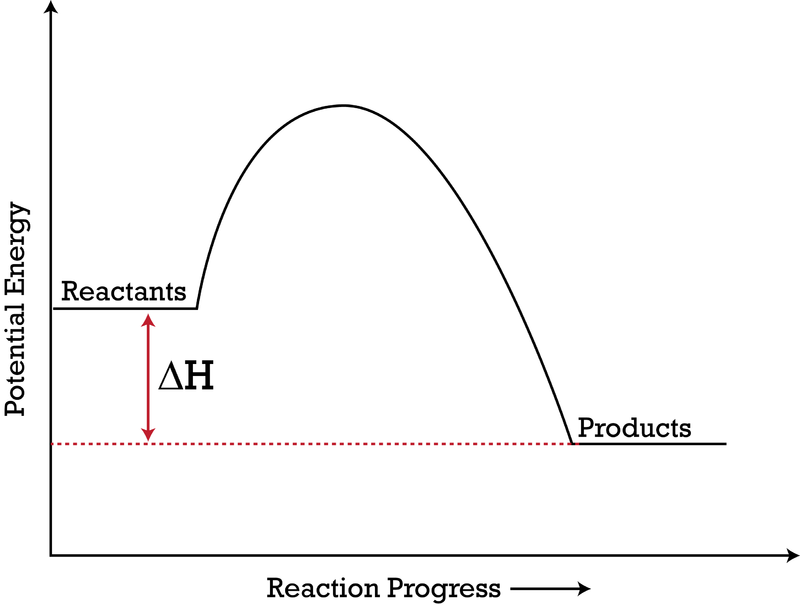
How to draw the potential energy diagram for this reaction ... The reaction in question is exothermic (releases heat) hence its products shall have chemical potential energies lower than that of its reactants- some of the potential energies have been converted to thermal energy during the reaction process. The enthalpy of a system, H, measures the sum of its internal and potential energy.
courses.lumenlearning.com › cheminter › chapterPotential Energy Diagrams | Chemistry for Non-Majors A potential energy diagram shows the change in potential energy of a system as reactants are converted into products. The figure below shows basic potential energy diagrams for an endothermic (A) and an exothermic (B) reaction. Recall that the enthalpy change is positive for an endothermic reaction and negative for an exothermic reaction.
Welcome to CK-12 Foundation | CK-12 Foundation Look at the two exothermic reactions whose potential energy diagrams are represented in figures A and B below, and notice the activation energy marked in each. When a reaction has a low activation energy, like in figure A above, most of the reactant molecules have sufficient kinetic energy to react, and the reaction will most likely be rapid (a ...
Endothermic and Exothermic Activity.pdf - Endothermic and ... Endothermic and Exothermic Activity For this assignment, you will create your own potential energy diagrams for each of the three chemical reactions. Then you will analyze the data and your diagrams for each reaction. Generic Reactions Reactants Products Transition State Synthesis A + B → AB A + B −15 kJ AB 20 kJ 30 kJ Single Replacement C + AB → CB + A C + AB 65 kJ CB + A 30 kJ 85 kJ ...
The diagram shows the potential energy changes for a ... Since the potential energy of the products is higher than the potential energy of the reactants, the reaction is endothermic and the enthalpy (E(C)-E(A)) has a positive sign.The activation energy (E(B)-E(A)) is positive as well.. We have a diagram showing the potential energy changes for a reaction pathway.. Point A represents the potential energy of the reactants.
en.wikipedia.org › wiki › EnergyEnergy - Wikipedia The total energy of a system can be subdivided and classified into potential energy, kinetic energy, or combinations of the two in various ways. Kinetic energy is determined by the movement of an object – or the composite motion of the components of an object – and potential energy reflects the potential of an object to have motion, and generally is a function of the position of an object ...
chemed.chem.purdue.edu › genchem › topicreviewGibbs Free Energy - Purdue University Reactions are classified as either exothermic (H < 0) or endothermic (H > 0) on the basis of whether they give off or absorb heat. Reactions can also be classified as exergonic (G < 0) or endergonic (G > 0) on the basis of whether the free energy of the system decreases or increases during the reaction.
Solved Which potential energy diagram represents an ... Question: Which potential energy diagram represents an exothermic reaction? Potential Energy Potential Energy non Reaction coordinate A) Reaction coordinate B) Reaction coordinate C) Reaction coordinate D) Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer. a a b b с c d d Which potential ...
PDF Topic 5.1 Exothermic and Endothermic Reactions Heat and ... Decrease in kinetic energy - energy released - exothermic Energy level diagrams Chemical Potential Energy The chemical potential energy stored in the bonds gives us a measure of a substances energy level. The higher the energy, the more chemical energy is stored in its bonds. The reactants and products
Which statement is true about the potential energy diagram ... Answer: Products have less potential energy than reactants. In an endothermic reaction, the reaction mixture will absorb heat from the surroundings. Therefore, the products will have higher energy than the reactants and hence, ΔH will be positive. In an exothermic reaction, the reaction mixture will release heat to the surroundings.
Heat Of Reaction Amd Potential Energy Diagram potential energy of the reactants is equal to the (1) heat of fusion (2) heat of reaction (3) activation energy of the forward reaction (4) activation energy of the reverse reaction 5 The energy absorbed and the energy released during a chemical reaction are best represented by a (1) cooling curve (2) heating curve (3) kinetic energy diagram
socratic.org › questions › 577fcfdc11ef6b60ad515f59What is the activation energy for the reverse reaction in ... Aug 03, 2016 · This is asking you to draw a potential energy diagram for an endothermic reaction. Recall that DeltaH_"rxn", the enthalpy of reaction, is positive for endothermic reactions, i.e. the product(s) (right) are higher in energy than the reactant(s) (left) and energy was absorbed. (Energy increases from bottom to top.) Since... the activation energy for the forward reaction is the difference in ...
estebantorreshighschool.com › equation-help › how-toHow to tell if a reaction is exothermic or endothermic from ... Image of a graph showing potential energy in relation to the process of a chemical reaction. In the case of an exothermic reaction, the reactants are at a higher energy level as compared to the products, as shown below in the energy diagram. In other words, the products are more stable than the reactants.




















0 Response to "42 potential energy diagram exothermic"
Post a Comment