40 orbital diagram for ne
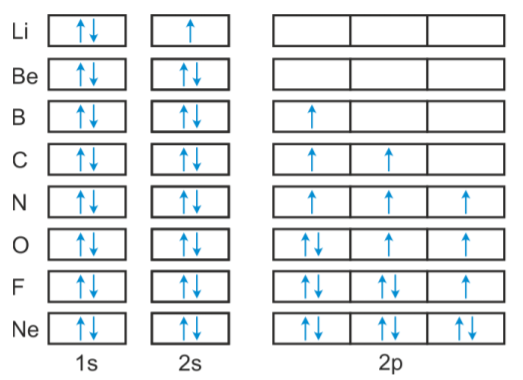
Orbital diagram of Nitrogen (N) 8. Orbital diagram of Oxygen (O) 9. Orbital diagram of Fluorine (F) 10. Orbital diagram of Neon (Ne) 11. Orbital diagram of Sodium (Na) Neon (Ne) has an atomic mass of 10. Find out about its chemical and physical ... Electron Configuration, [He] 2s2 2p6. 1s2 2s2 2p6. Orbital Diagram.
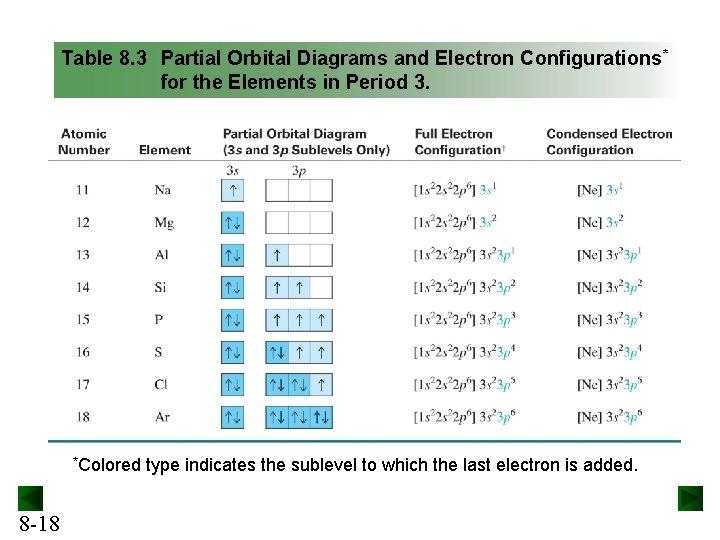
condensed electron configuration: [Ne] 3s 2. orbital diagram (orbital box diagram) : Pairs of electrons occupy the 1s, 2s, 2p x, 2p y and 2p z boxes, with 2 electrons placed in the 3s box Apply the Pauli Exclusion Principle so that for paired electrons, one electron has "up spin" and the other has "down spin" ↑↓ ↑↓ ↑↓ ↑↓

Orbital diagram for ne
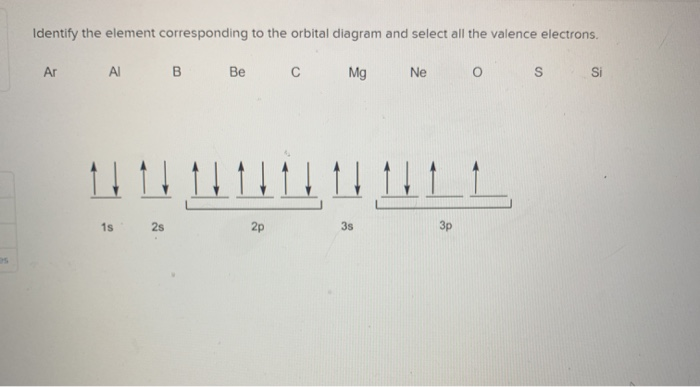
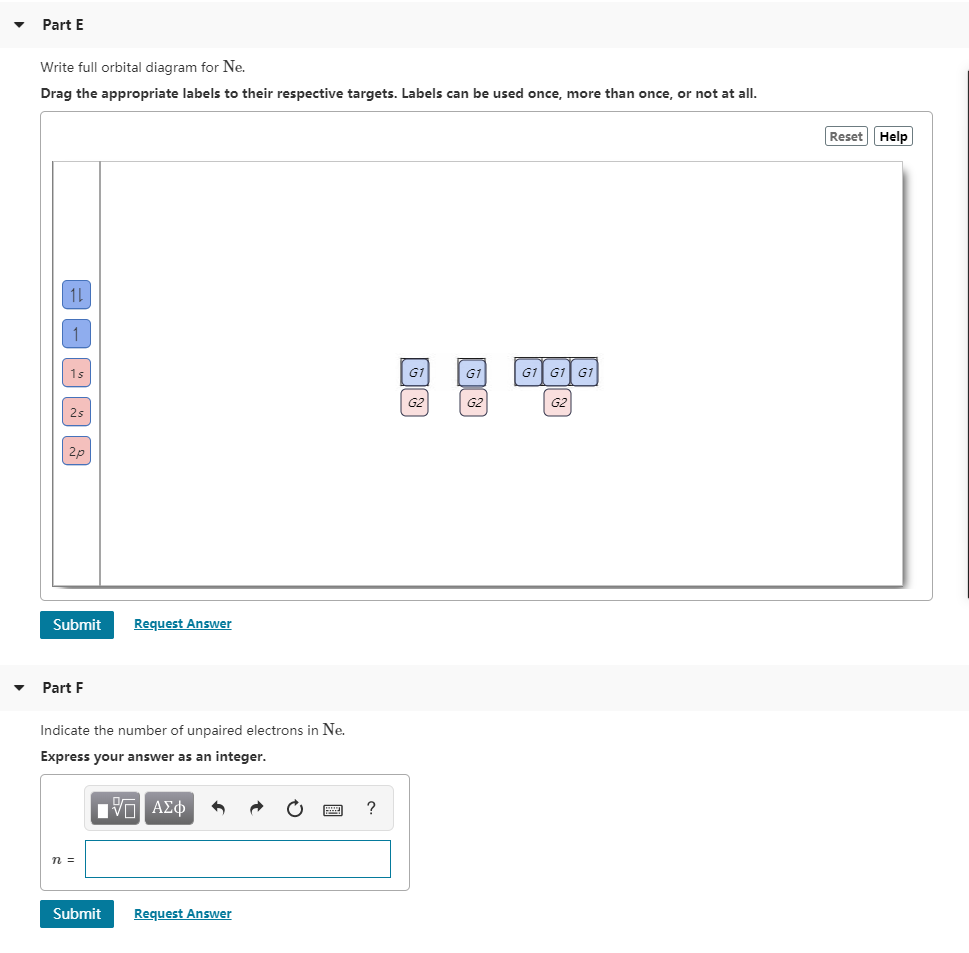
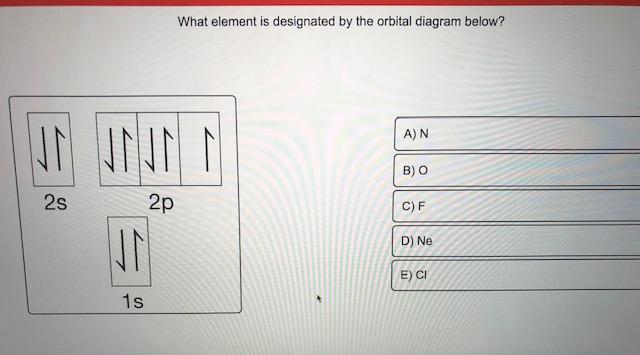
0:21 Molecular Orbital Diagram of Oxygen Molecule3:30 Molecular Orbital Diagram of Florine Molecule5:25 Molecular Orbital Diagram of Neon MoleculeSo as we d... A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of ... - Partc Write orbital diagrams for the valence electrons of I Drag the appropriate; Question: Part A Write orbital diagrams for the valence electrons of Ne. Drag the appropriate labels to their respective targets. Not all labels will be used. 03 QORIDODOO G2 Part B Indicate the number of unpaired electrons in Ne. Express your answer as an Integer.
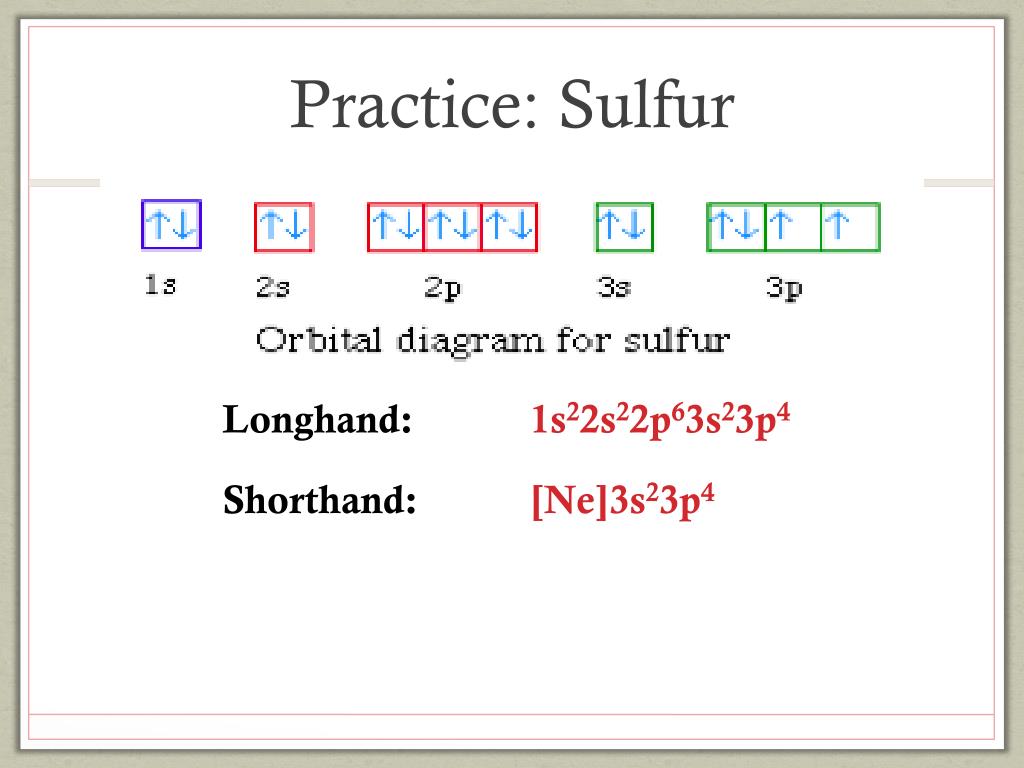
Orbital diagram for ne. What is the orbital diagram for Helium? Helium only has 2 electrons and therefore it has a configuration of 1s2. Because the 1s orbital is full with 2 electrons and any additional electrons would go in a new energy level. The electron configuration for Helium shows a full outer shell and is Helium is therefore called a Nobel Gas. Chemistry questions and answers. Use the electron arrangement interactive to practice building electron arrangements Identify the element that corresponds to the orbital diagram.1s' 252 2p 382 3p O Si O AI O Ne O c 3s廾 2s # is # Create the orbital diagram for sodium. Answer Bank 3s 2p 2s 1s. Sodium electron configuration is 1s 2 2s 2 2p 6 3s 1.The symbol for sodium is 'Na'. The period of sodium is 3 and it is a s-block element. This article gives an idea about the electron configuration of sodium(Ne) and orbital diagrams, period and groups, valency and valence electrons of sodium, bond formation, compound formation, application of different principles. The 1s orbital at the bottom of the diagram is the orbital with electrons of lowest energy. The energy increases as we move up to the 2 s and then 2 p , 3 s , and 3 p orbitals, showing that the increasing n value has more influence on energy than the increasing l value for small atoms.
Answer to: (a) Write the full orbital diagram for Ne. (b) Indicate the number of unpaired electrons in it. By signing up, you'll get thousands of... MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n... Answer and Explanation: 1. For neon, the atomic number is 10. The electronic configuration for neon is 1s22s22p6 1 s 2 2 s 2 2 p 6 . The full orbital diagram for neon is shown below. Orbital diagram.
Even rather simple molecular orbital (MO) theory can be used to predict from the bottom of the diagram because this is how MO diagrams are constructed, from N2, O2, F2, Ne2 the complexity of the molecular orbitals develop in two ways.Draw the molecular orbital diagram for Ne 2 + and determine if the bond between the two atoms will be stable. Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics. Since 1s can only hold two electrons the next 2 electrons for Ne go in the 2s orbital. The remaining six electrons will go in the 2p orbital. Therefore the Ne electron configuration will be 1s 2 2s 2 2p 6. Because the second energy level (2s 2 2p 6) has eight electrons Neon has an octet and has a full outer shell. It is therefore a Nobel Gas. [Ne]3s2 [Ar]4s13d1 [Ne]3s23p6 [Ar]4s2 [Ar]3d2 [Ar]4s2. Consider the element with the electron configuration [Kr]5s24d7. This element is an actinide element. ... The orbital diagram for a ground-state nitrogen atom is 1s 2s 2p A ↿⇂ ↿⇂ ↿ . ↿ . ↿ B ↿⇂ ↿ . ↿⇂ ↿ .
Orbital Diagram For Nitrogen (N) | Nitrogen Electron Configuration February 15, 2021 by Sneha Leave a Comment Nitrogen Electron Configuration : When we talk about school subjects, then one of the major subjects which are very important for knowledge perspective is science .
•To complete an orbital diagram using arrows to represent electrons . Where are Electrons? •Electrons exist in different energy levels ... [Ne] = 1s 22s 2p6 •[Ar] = 1s22s22p63s23p6 •Potassium -Atomic Number = 19 -1s22s 22p63s 3p64s1 -[Ar]4s1. Pauli Exclusion Principle
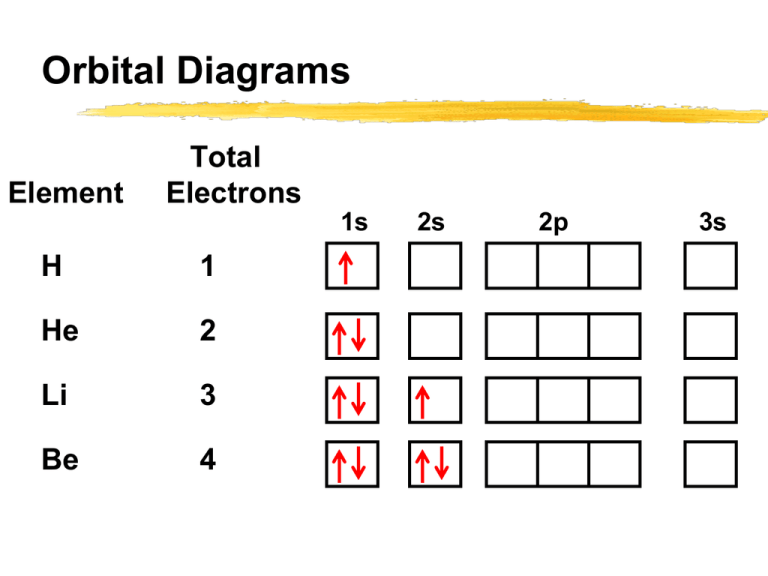
1. The Pauli principle: No more than two electrons can occupy a given orbital. If there are two electrons in an orbital, their spins must be paired (one must have m s = 1 2 and the other, m s = − 1 2). 2. The aufbau (building-up) principle: When electrons are filled in to orbitals in an atom, the orbitals with lower energy are filled first.
The 1s orbital at the bottom of the diagram is the orbital with electrons of lowest energy. The energy increases as we move up to the 2 s and then 2 p , 3 s , and 3 p orbitals, showing that the increasing n value has more influence on energy than the increasing l value for small atoms.
Jan 21, 2021 — The electron configuration of the Ne can write as 1s22s22p6. It simply depicts the theory that the first two orbital the 1s and the 2s are ...
[Ne] 1s1 Let's calculate the abbreviated electron configuration for calcium (atomic number 20). Calcium is in row 4 of the periodic table. The inert gas in row three is Argon, atomic number 18. Now we have 2 more electrons to place into orbitals.
Molecular Orbital Diagrams, Bond Order, and Number of Unpaired Electrons Draw the molecular orbital diagram for the oxygen molecule, O 2. From this diagram, calculate the bond order for O 2. How does this diagram account for the paramagnetism of O 2? Solution. We draw a molecular orbital energy diagram similar to that shown in Figure 11.
Orbital Diagram 1s ↿⇂ 2s ↿⇂ 2p ↿⇂ ↿⇂ ↿⇂ 3s ↿⇂ 3p ↿⇂ ↿⇂ ↿⇂ 3d ↿⇂ ↿⇂ ↿⇂ ↿⇂ ↿⇂ 4s ↿⇂ 4p ↿ ↿ ↿ 4d 4f ... to that atom, followed by the configuration of the remaining electrons. So for sodium, we make the substitution of [Ne] for the 1s22s22p6 part of the configuration. Sodium's ...
Nov 7, 2021 — We use the orbital energy diagram of Figure 6.8.1, ... For example, [Ne] represents the 1s22s22p6 electron configuration of neon (Z = 10), ...

Draw The Partial Valence Level Orbital Diagram And Write The Symbol Group Number And Period Number Of The Element Ar 4s 2 3d 10 4p 3 Image Src Orbital9195593143458043682 Jpg Alt Orbital C Study Com
Neon electron configuration is 1s 2 2s 2 2p 6.The symbol for neon is 'Ne' and it is an inert element. This article gives an idea about the electron configuration of neon(Ne) and orbital diagram, period and groups, valency and valence electrons of neon, application of different principles. The tenth element in the periodic table is neon.
Molecular Orbital Diagrams simplified. Megan Lim. Oct 26, 2016 · 3 min read. Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The first major step is understanding ...
Orbital Filling Diagrams •Each box represents an orbital which can hold a max of 2 e- •Aufbau principal -each electron occupies the lowest energy orbital available; German for "build up" •Electrons are notated with an arrow -Up arrow goes first then, down arrow -Arrows represent the opposing spin of electrons 5.2 Quantum Theory & The Atom
Molecular Orbital Diagrams of Diatomic Molecules Introduction: In chemistry molecular orbital (MO) theory is a method for determining molecular structure in which electrons are not assigned to individual bonds between atoms, but are treated as moving under the influence of the nuclei in the whole molecule.
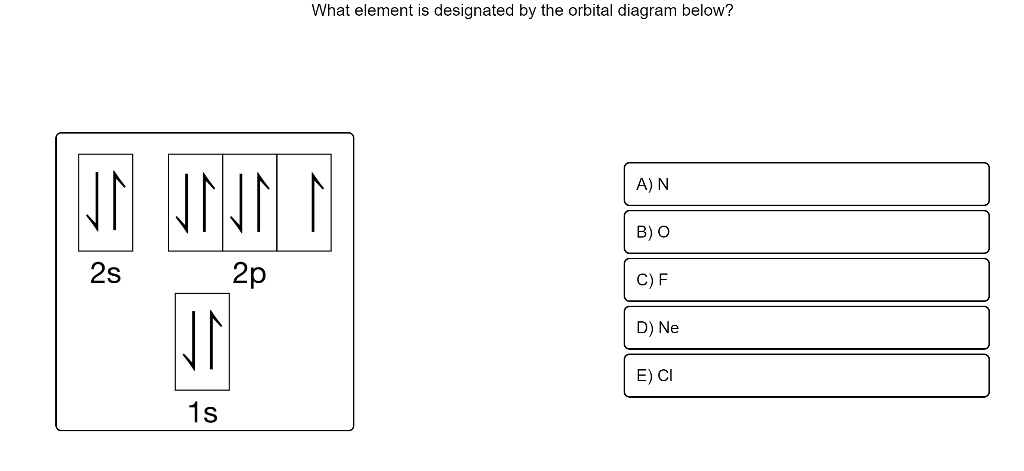
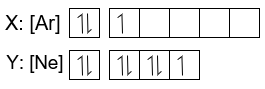
Terms in this set (20) How many boxes are required to depict the 4f orbitals? 7. The orbital diagram represents what element? (1s2 2s2 2p6 3s2 3p6 4s) Potassium. What atom will have unpaired 2p electrons? O (oxygen) Orbitals that have the same energy are called?
- Partc Write orbital diagrams for the valence electrons of I Drag the appropriate; Question: Part A Write orbital diagrams for the valence electrons of Ne. Drag the appropriate labels to their respective targets. Not all labels will be used. 03 QORIDODOO G2 Part B Indicate the number of unpaired electrons in Ne. Express your answer as an Integer.
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of ...

Electron Configurations Distributedexplains How Electrons Are Distributed Among An Atom S Orbitals Address Each Part Identifies Part Of An Electron S Address Ppt Download
0:21 Molecular Orbital Diagram of Oxygen Molecule3:30 Molecular Orbital Diagram of Florine Molecule5:25 Molecular Orbital Diagram of Neon MoleculeSo as we d...

Show The Orbital Diagrams For The Following Chromium Atom And Chromium Iii Ion Include Only Occupied Orbital S A Cr B Cr 3 Study Com




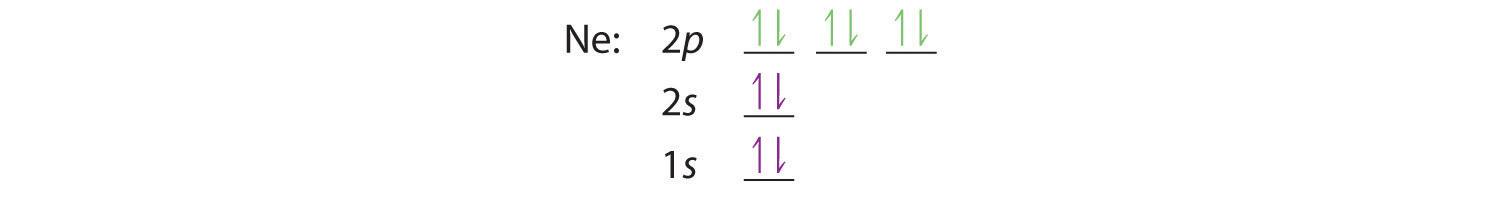




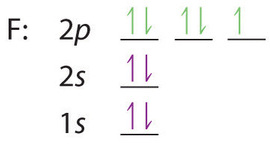
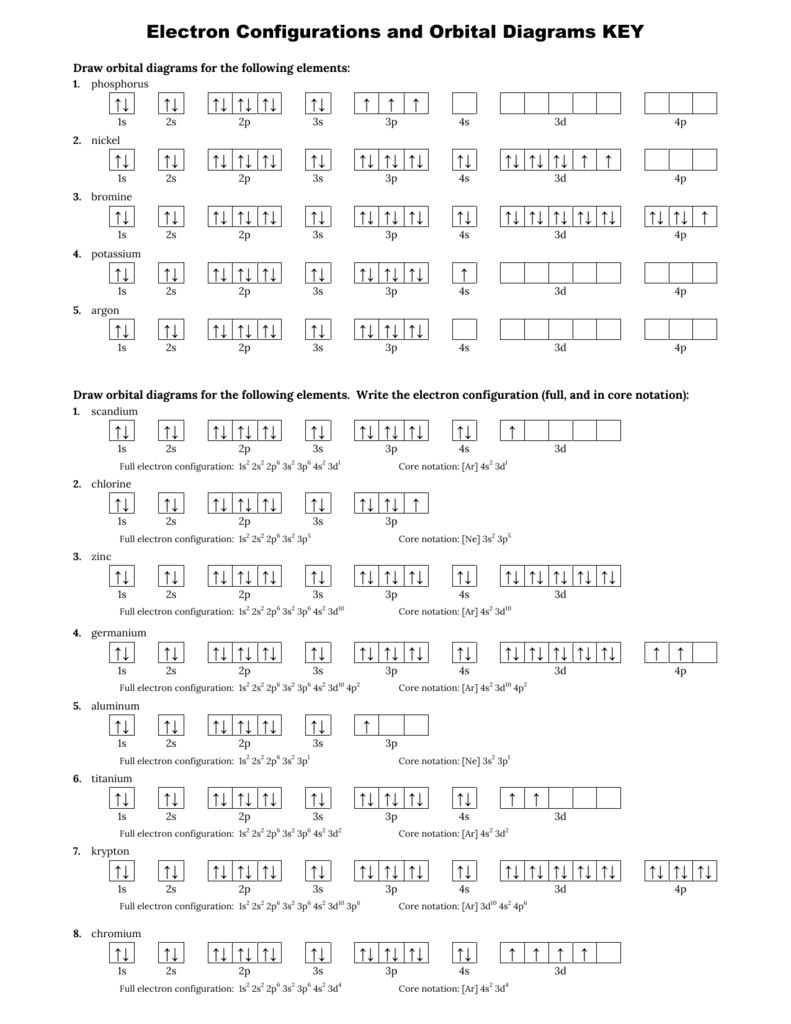





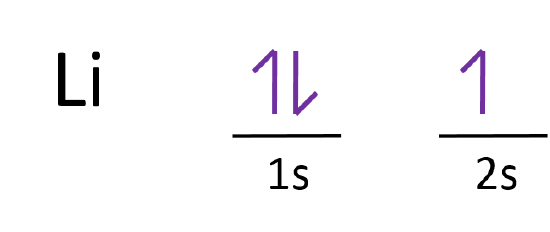
0 Response to "40 orbital diagram for ne"
Post a Comment