41 orbital diagram for ti2+
Carbon monoxide electron configuration 17.09.2021 · In this work, Ti3C2, which has a loosely packed accordion-like structure in transition metal carbide (MXene) form, is fabricated and adsorbed by three metal ions (Fe3+/Co2+/Ni2+). The electromagnetic interference (EMI) shielding performance of Ti3C2 and Ti3C2:Fe3+/Co2+/Ni2+ films is researched in detail, demonstrating that the EMI shielding effectiveness can be improved by …
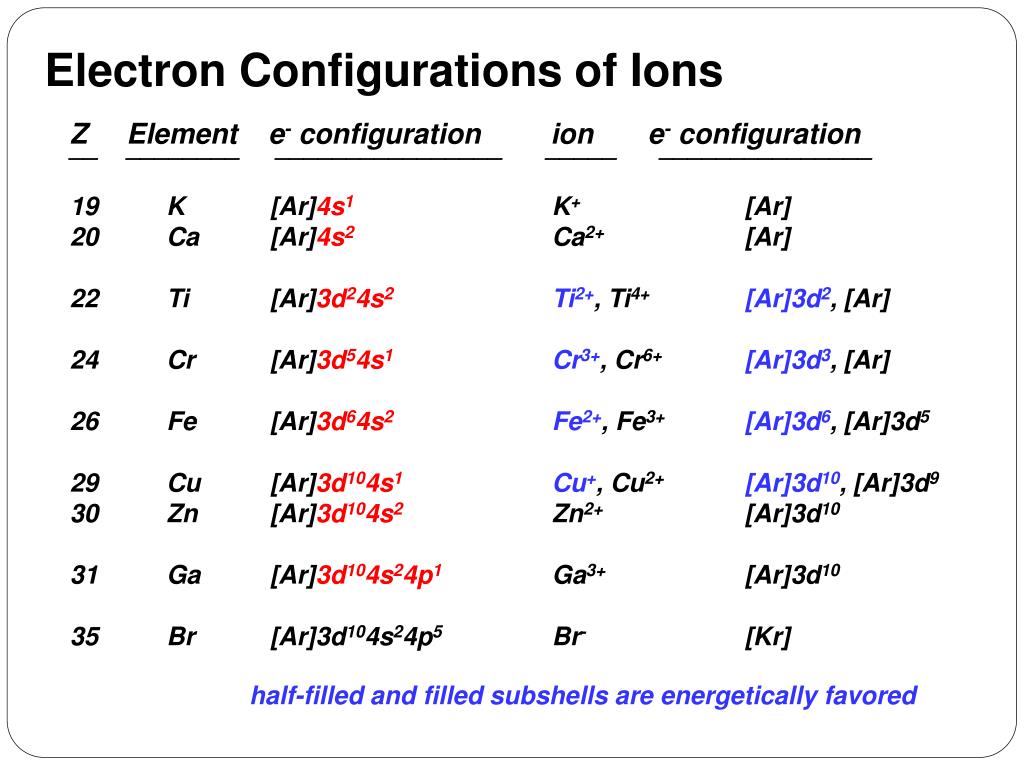
Jun 27, 2016 · 1 answerTi2+:[Ar]3d2. Explanation: A good place to start when trying to figure out the electron configuration of an ion is the electron ...
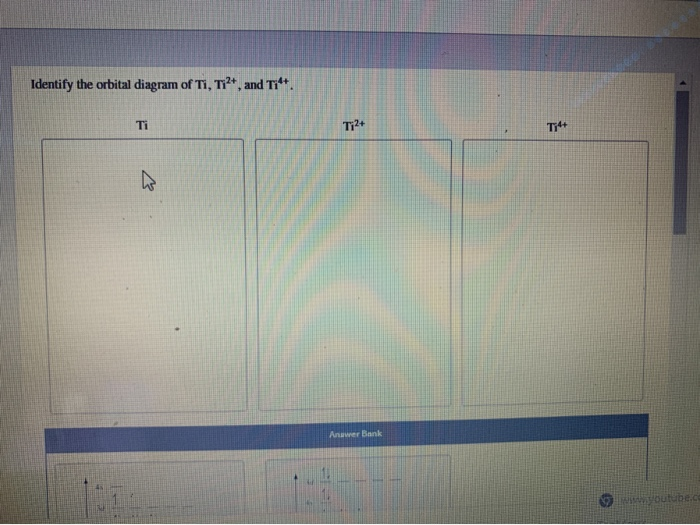
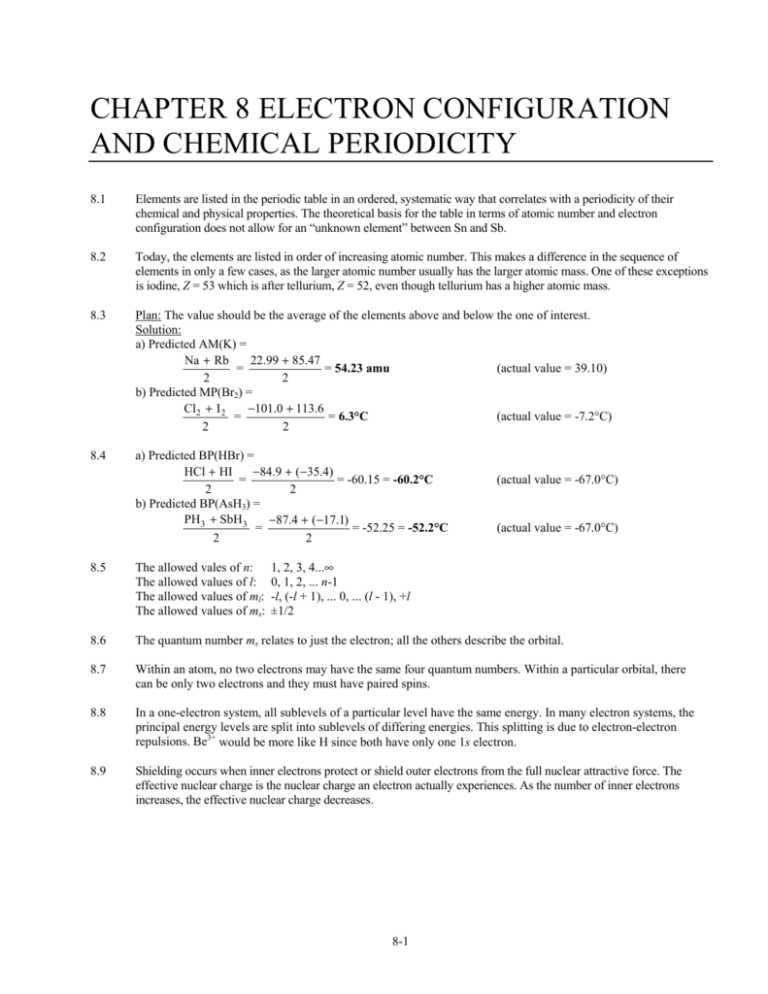
Orbital diagram for ti2+
19.03.2021 · Polarons are quasiparticles that easily form in polarizable materials due to the coupling of excess electrons or holes with ionic vibrations. These quasiparticles manifest themselves in many ... Academia.edu is a platform for academics to share research papers. Orbital Diagrams. 4 videos ... Electron Configuration. 3 videos ... Now if you take Ti2+, the ion, basically you're taking away two electrons.Feb 7, 2014
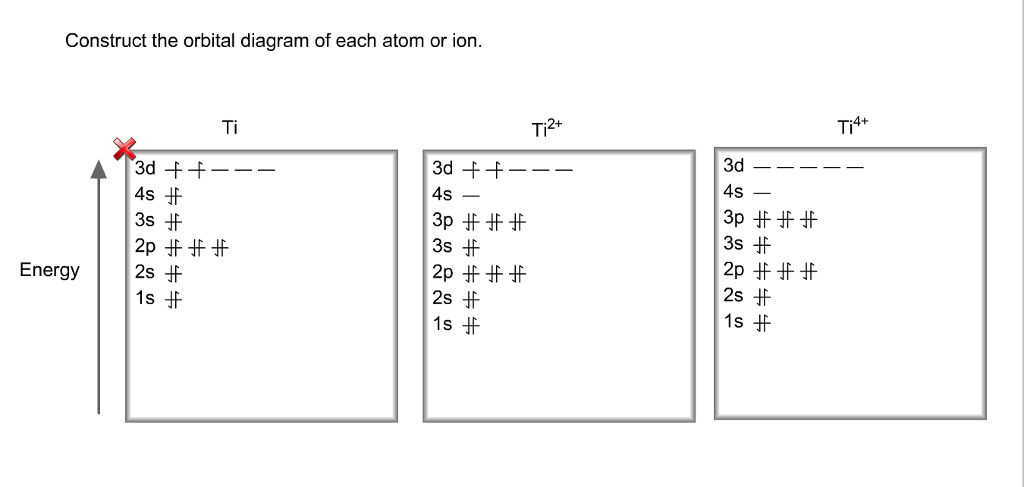
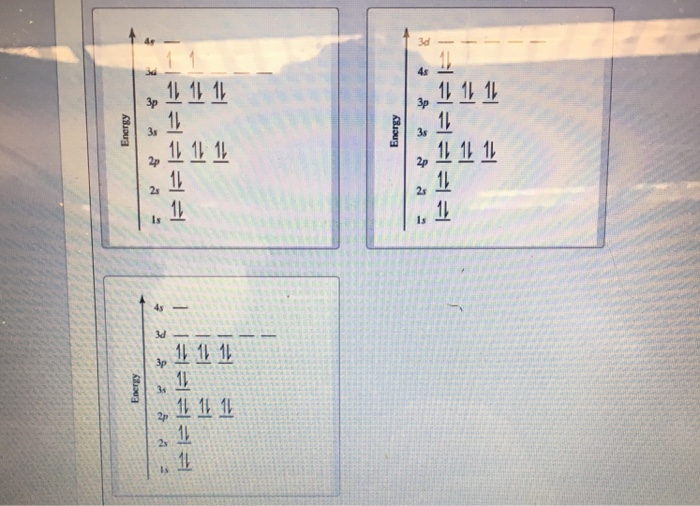
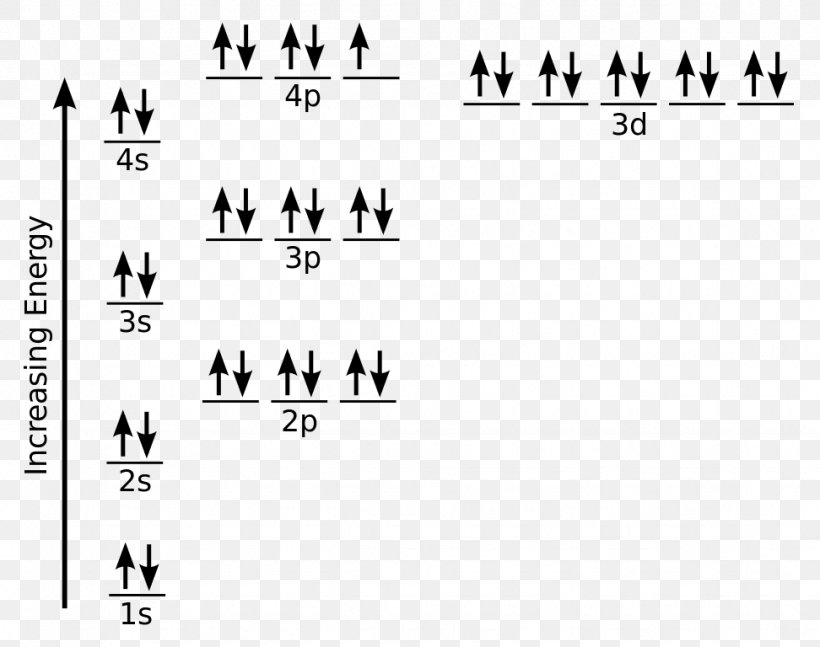
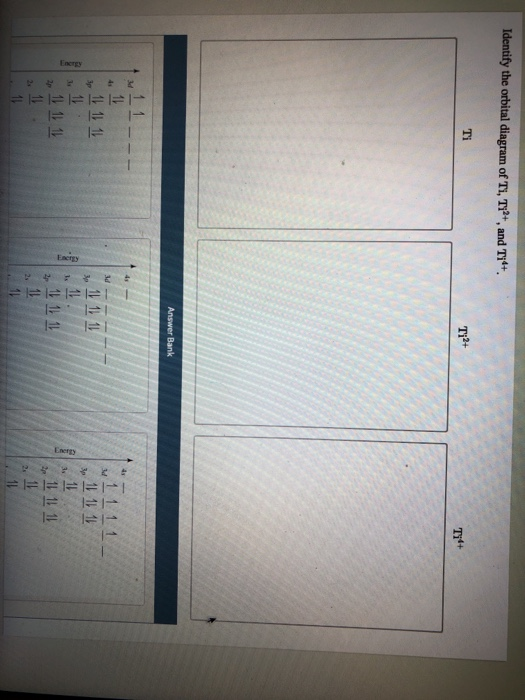
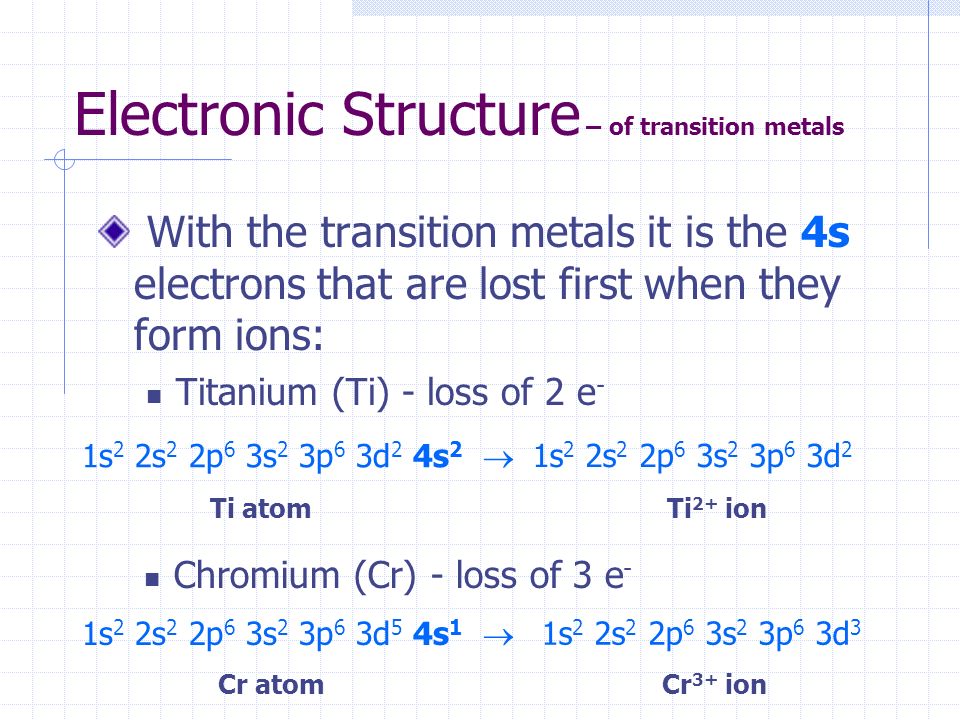
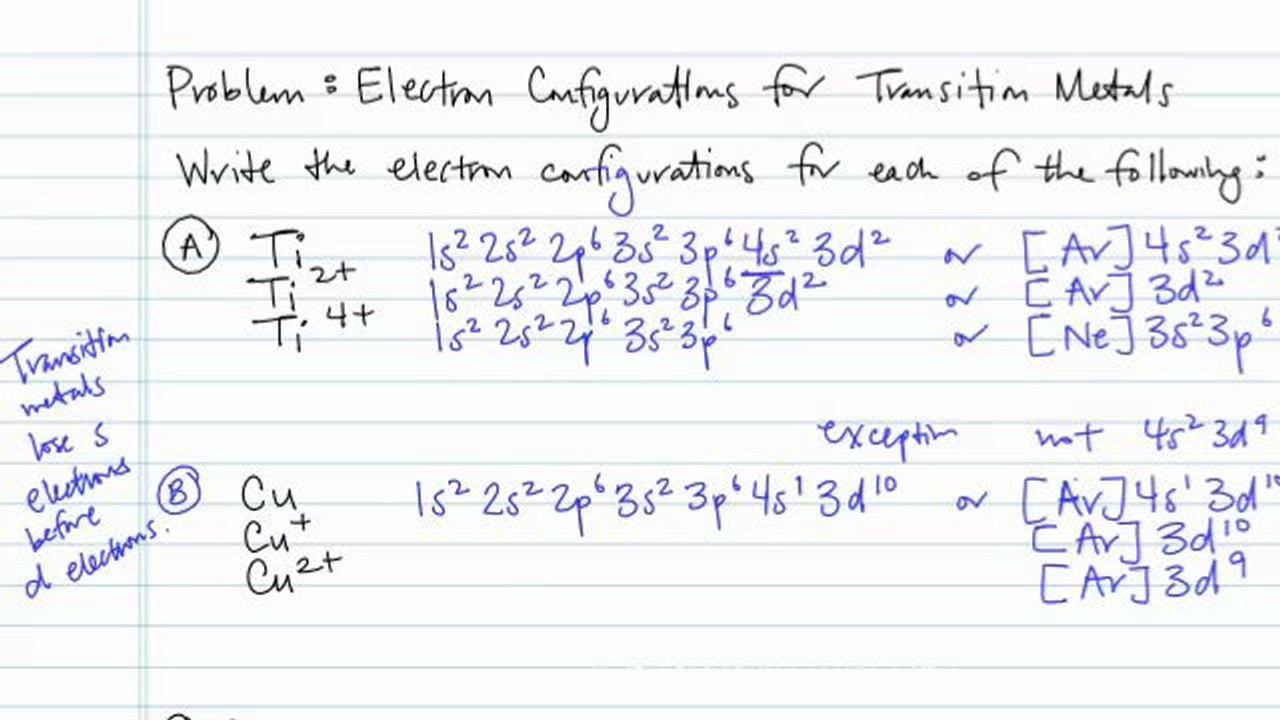
Orbital diagram for ti2+. This problem has been solved! ... Construct the orbital diagram of each atom or ion. Ti. Ti2+. Ti4+. Expert Answer. Note that the 4s electrons are removed before the 3d electrons, so the electron configuration of Ti2+ would be [Ar]3d2 not [Ar]4s2. core electrons valence ... B) An orbital that penetrates into the region occupied by core electrons is more shielded from nuclear charge than an orbital that does not penetrate and therefore has a lower energy. C) It is possible for two electrons in the same atom to have identical values for all four quantum numbers. D) Two electrons in the same orbital can have the same ... According to the Aufbau principle, the energy of the 3d is slightly more than the 4s orbital so, electrons will first fill the 4s shell.
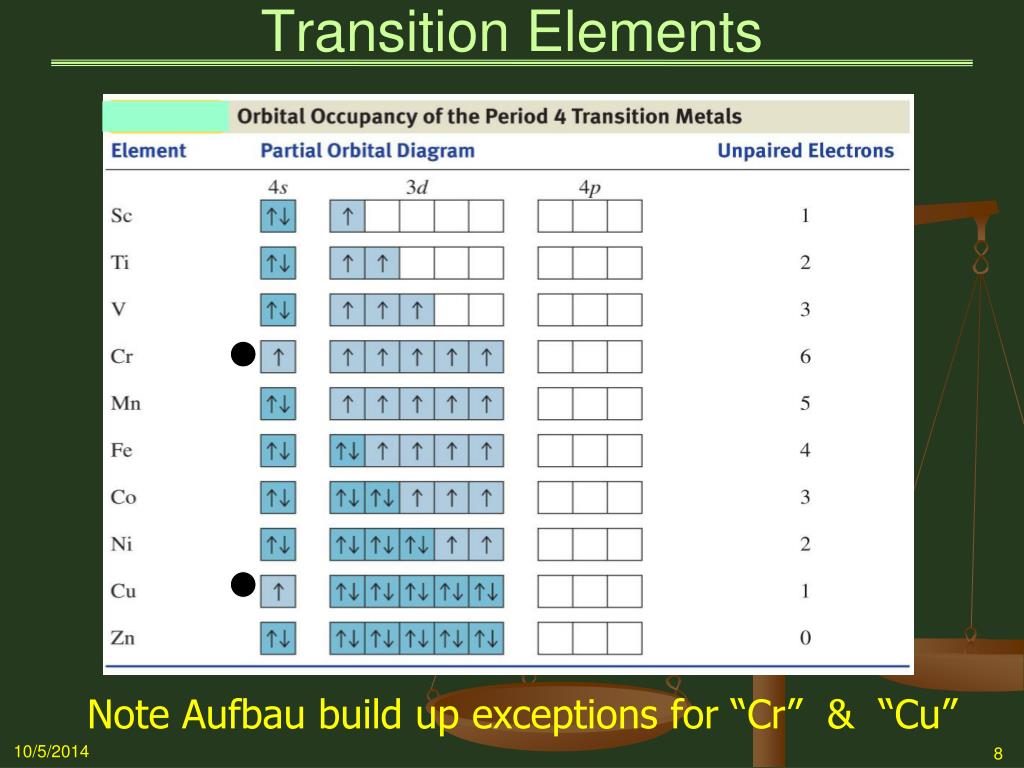
Ap physics 1 energy ppt Construct the orbital diagram of each atom or ion. Ti. Ti2+. Ti4+. Learn this topic by watchingThe Electron Configuration: Ions ...Jan 21, 2019 by SM Jacobsen · 1988 · Cited by 49 — From an optical spectroscopic viewpoint Ti2+, having a 3d2 electron configuration, is very interesting, because it is the system treated in detail in almost ... Academia.edu is a platform for academics to share research papers.
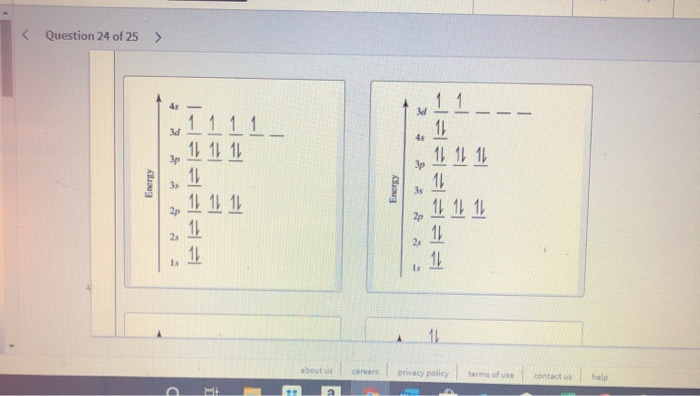
Quantum Number Questions and Answers. Get help with your Quantum number homework. Access the answers to hundreds of Quantum number questions that are explained in a way that's easy for you to ... When drawing an orbital diagram, orbitals of _____ energy are filled first. By convention, the _____ electron in a given orbital is designated as ↑ and the direction of the arrow indicates the electron _____. 1 answerThe electron configuration of a neutral titanium atom is. Ti: 1s2 2s2 2p6 3s2 3p6 4s2 3d2. The two electrons that are lost when the Ti2+ is formed will come ... Complete Solutions Manual General Chemistry Ninth Edition ... - ID:5dcdb97adce08. Complete Solutions Manual GENERAL CHEMISTRY NINTH EDITION Ebbing/Gammon. Uploaded by. Sofia Uribe Sanchez. connect to do...
Orbital Diagrams. 4 videos ... Electron Configuration. 3 videos ... Now if you take Ti2+, the ion, basically you're taking away two electrons.Feb 7, 2014
Academia.edu is a platform for academics to share research papers.
19.03.2021 · Polarons are quasiparticles that easily form in polarizable materials due to the coupling of excess electrons or holes with ionic vibrations. These quasiparticles manifest themselves in many ...



















0 Response to "41 orbital diagram for ti2+"
Post a Comment