40 ti2+ orbital diagram
Choose the ground state electron configuration for Ti2+ ... Choose the valence electron orbital diagram that represents the ground state of Se2-D (#1) The solid compound K2S2O3 contains. K+ ions and S2O3^2-Of the following, which atom has the largest atomic radius? K. Choose the best Lewis structure for SF4. E (#28)
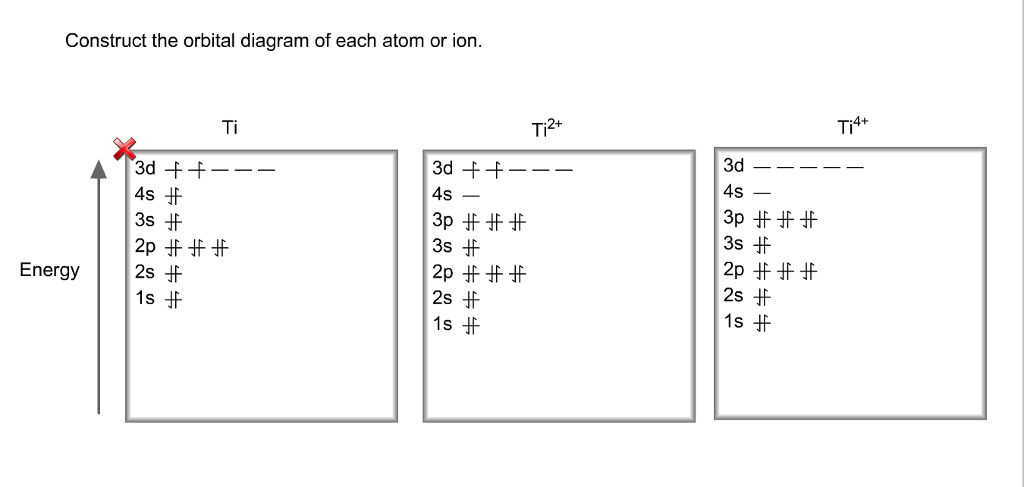
Now if it's Ti4+, now we've taken away two additional electrons compared to the Ti2+. So we would write out 1s2, 2s2, 2p6, 3s2, 3p6, and then the 3d2-electrons would be gone. So compared to the original Titanium atom, we gave away four electrons. So both the 4s2, and the 3d2 electrons would be gone.
40 orbital diagram for ti. By knowing any two values of the Voltage, Current or Resistance quan titi es we can use Ohms Law to find the third missing value.Ohms Law is used extensively in electronics for mulas and calcula ti ons so it is "very important to understand and accurately remember these for mulas"..

Ti2+ orbital diagram
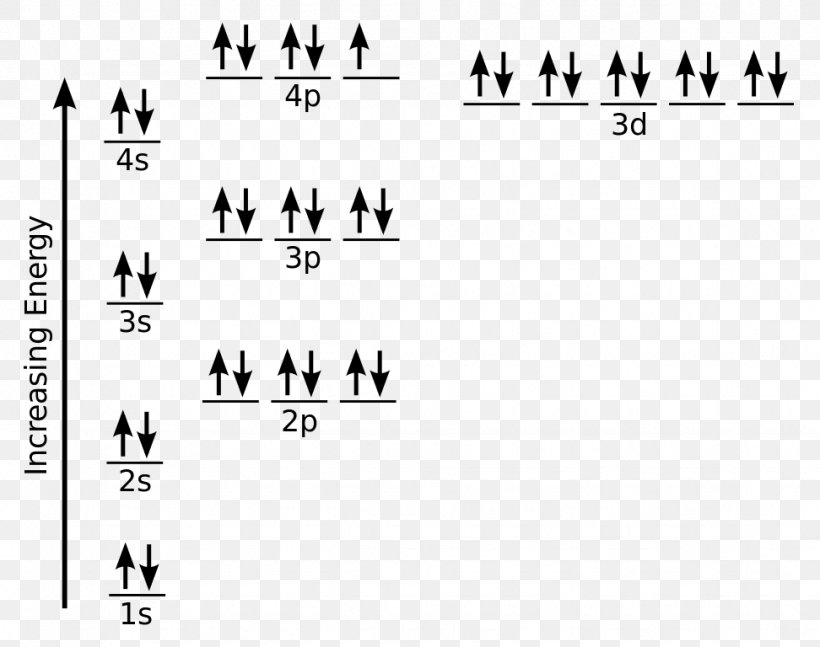
Orbital Diagram Of Ti2+ Answer to Construct the orbital diagram of each atom or ion. Ti Ti2+ Ti4+. Figure A vertical orbital diagram for the Li ground state. .. Ti2+ has 2 unpaired electrons and is paramagnetic, providing evidence that the 4s electrons .
Orbital Diagram for Ti2 electron configuration for the titanium ion ti2 this video shows you how to write the electron configuration for the titanium ion ti 2 electron orbitals question 111 so if you count the electrons of the ti2 in the s orbital you need to know hund s rule and specifically this diagram electron orbitals This is only a preview
Titanium Electron Configuration (Ti) with Orbital Diagram. Titanium Electron Configuration: Titanium is a chemical element that has a chemical symbol Ti. Its atomic number is 22. It is a transition metal lustrous which has a silver colour, low density, and high strength. It is resistant to corrosion in aqua regia, sea water, and chlorine.
Ti2+ orbital diagram.
This means that when titanium loses electrons, it does so from the 4s orbital first. Ti: 1s22s22p63s23p63d24s2 Therefore, the two electrons that are lost when the Ti2+ is formed will come from the 4s orbital, which means that the electron configuration of the cation is
Figure A vertical orbital diagram for the Li ground state. .. Ti2+ has 2 unpaired electrons and is paramagnetic, providing evidence that the 4s electrons . Which free ion has the greater number of unpaired d electrons, Ti2+ or Co2+? Draw the orbital diagram for the d orbitals in an octahedral complex containing.
Section 1.6 - 4 • A general term symbol that uniquely describes a specific electronic configuration looks like this: (2S+1)L J where 2S + 1 is the spin multiplicity (and S is the total spin angular momentum.) L is the total orbital angular momentum J is the total angular momentum (spin + orbital) S = 0 → "Singlet" S = ½ → "Doublet" S = 1 → "Triplet" etc.
This video shows how to draw the orbital diagram of Titanium (Ti). It also shows how to write the electron configuration of titanium and the shorthand noble...
Ti2+ has 2 unpaired electrons and is paramagnetic, providing evidence that the 4s electrons . When filling degenerate orbital s, electrons fill the m singly first, with parallel spins is ... Electron orbital diagram s and written configurat ion s tell you which orbital s are filled and which are partially filled for any atom.
Future Publishing/Future/Getty Images. The electron configuration for titanium is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 2 4s 2, according to the Jefferson Lab website. The element's 22 electrons are arranged in four energy levels surrounding the nucleus of the atom.
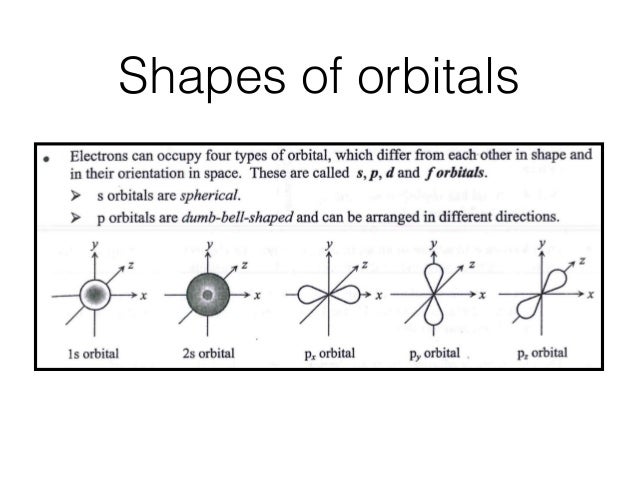
28. For a multielectron atom the energy differences between the s,p,d,and f orbitals is due to. Electron-electron repulsions. Which of the following is NOT a valid set of quantum numbers? N+3,l=2, ml=3, ms=1/2. What are the possible values of n and ml for an electron in a 5d orbital? n=5 and ml=-2,-1,0,1,2.
Problem Details. Construct the orbital diagram of each atom or ion. Ti. Ti 2+. Ti 4+. Learn this topic by watching The Electron Configuration: Ions Concept Videos.
Diagram Orbital Atom Nikel. Untuk menentukan electron yang tidak berpasangan cukup membuat diagram orbital dari subkulit yang diisi electron tidak penuh yaitu 3d yang terisi 8 elektron. Subkulit d terdiri 5 orbital yang dapat ditempati oleh 10 elektron maksimum. Jumlah elektron tidak berpasangan pada subkulit 3d dapat dilihat pada gambar berikut
What is the orbital diagram for Ti 2+? I got 1s two arrows, 2s two arrows, 2p 6 arrows, 3s two arrows, 3p six arrows, 4s two arrows but it is wrong. It said ions of d-block metals typically lack the outermost s electrons that are present in their neutral counterparts.
To write the configuration for the Titanium ions, first we need to write the electron configuration for just Titanium (Ti). We first need to find the number...
This photo about: Orbital Diagram for Ti2, entitled as Sensors Free Full Text Orbital Diagram For Ti2 - also describes Sensors Free Full Text and labeled as: orbital diagram, with resolution 2399px x 1462px
Angular momentum l (orbital shape) Magnetic m l (orbital orientation) These 3 quantum numbers are the spatial quantum numbers. ⇒ together, they describe the 3D appearance of the orbital in space ⇒ the spatial probability distribution of an e-described by that orbital The 4th quantum number is necessary to fully describe an e-in an orbital.
Get the detailed answer: What is the orbital diagram of each atom or ion? Ti, Ti2+, Ti4+
Oxygen is the eighth element with a total of 8 electrons. In writing the electron configuration for oxygen the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for O go in the 2s orbital. The remaining four electrons will go in the 2p orbital. Therefore the O electron configuration will be ...
Answer to Solved Identify the orbital diagram of Ti, Ti2+, and Ti4+.
electrons into the same orbital •Πeis a stabilizing energy for electron exchange associated with two degenerate electrons having parallel spin total 3 e 0 c eg* t2g d4HS eg* t2g d8 eg* t2g d6LS total 7 e 3 c total 6 e 3 c LFSE 3 0.4 O 10.6 O 0.6 O LFSE 6 0.4 O 20.6 O
Orbital diagram for Sodium Now lets try one with ions! Electron configuration for N3-Because it has a charge of -3, there are three extra electrons to deal with. Normally, nitrogen has 7 electrons. But because of its charge, we have to add 3 more. If it has 10 electrons, write it out until you run out of electrons. 1s2 2s2 2p6
After the 4s is full we put the remaining six electrons in the 3d orbital and end with 3d9. Therefore the expected electron configuration for Copper will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 9 . Note that when writing the electron configuration for an atom like Cu, the 3d is usually written before the 4s.
The relative orbital levels for the hydrogen atom can be represented asDraw the relative orbital energy levels for atoms with more than one electron... Q. Using vertical lines, indicate the following transitionsa. n = 3 → n = 2b. n = 4 → n = 2c. n = 2 → n = 1on an energy-level diagram for the hydrogen...
Answer (1 of 2): Ti+2 in TiCl2 has 2 unpaired e-, while Ti+4 in TiO2 has no npaired e-s.So TiCl2 is paramagnetic while TiO2 is diamagnetic
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular. What is the orbital diagram for Ti 2+?
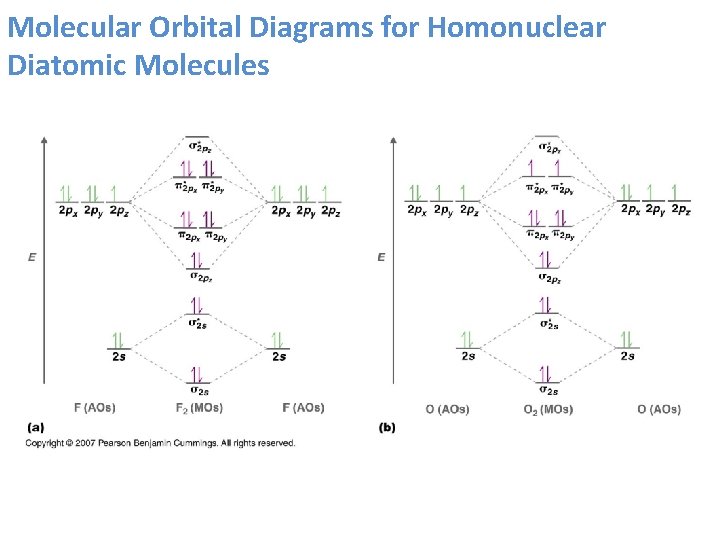
The atomic orbital of the O atoms overlap to form the sigma and pie orbital of the O2 molecules as shown in the diagram above. We add the 12 valence electron according to the aufbau principle. The last two electrons go into separate degenerate pie orbital. According to hund's rule. Thus,oxygen has two unpaired electrons and is paramagnetic.














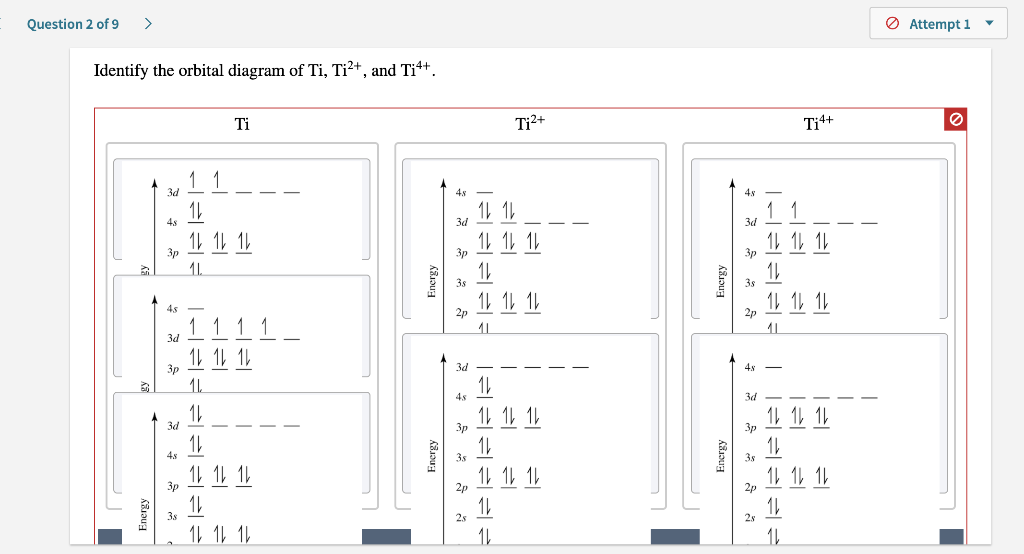


0 Response to "40 ti2+ orbital diagram"
Post a Comment