38 the cell diagram for the lead-acid
A good designed circuit of a Alkaline battery charger. The interesting thing of this circuit is that it uses a led that will show the charge of battery by blinking, when you connect a totally discharged battery the LED blink faster but when the battery charging process starts the LED blinking speed will dicrease slowly and completely stop when the battery will fully charged. Fuel cells and lead-acid batteries 10.626 (2011) Bazant. vertical lines. Reactions not involving H + such as (23) and (27) appear as horizontal lines. From reactions (28)-(29), we see that the Pb. 2+ ion is favored over metal-lic lead and lead oxide in acidic solutions and low potentials, and thus disso-
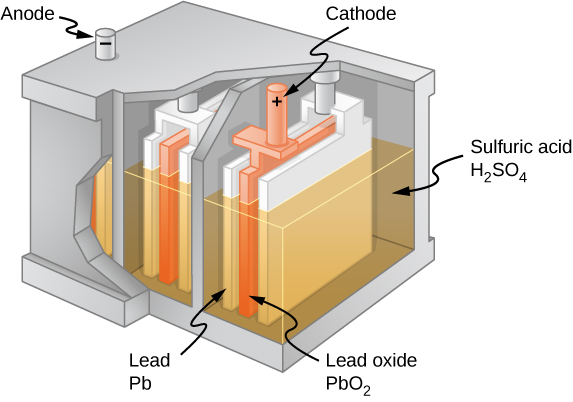
Tabbi Wilberforce, ... Abdul-Ghani Olabi, in Encyclopedia of Smart Materials, 2022. Lead Acid Batteries. Lead-Acid battery has been seen to be frequently in use for storage application (Malekshah et al., 2018).The components in Lead-Acid battery includes; stacked cells, immersed in a dilute solution of sulfuric acid (H 2 SO 4), as an electrolyte, as the positive electrode in each cells ...
The cell diagram for the lead-acid
The right-hand lead electrode is nonreactive. Write the balanced equation for the net cell reaction. equation: Look up standard potentials for the oxidation and reduction half-reactions, and then calculate the value of 𝐸∘cellEcell∘. 𝐸∘cell=Ecell∘=. VV. Calculate the value of Δ𝐺∘rxnΔGrxn∘. Δ𝐺∘rxn=ΔGrxn∘=. Plante plates or formed lead acid battery plates. Faure plates or pasted lead acid battery plates. Plante Plate Plante Process. In this process two sheets of lead are taken and immersed in dilute H 2 SO 4. When an current is passed into this lead acid cell from an external supply, then due to electrolysis, hydrogen and oxygen Feb 24, 2012 · A fully charged lead acid battery cell has voltage and specific gravity, 2.2 V and 1.250 respectively, and this cell is normally allowed to be discharged till the corresponding values become 1.8 V and 1.1 respectively. Maintenance of Lead Acid Battery
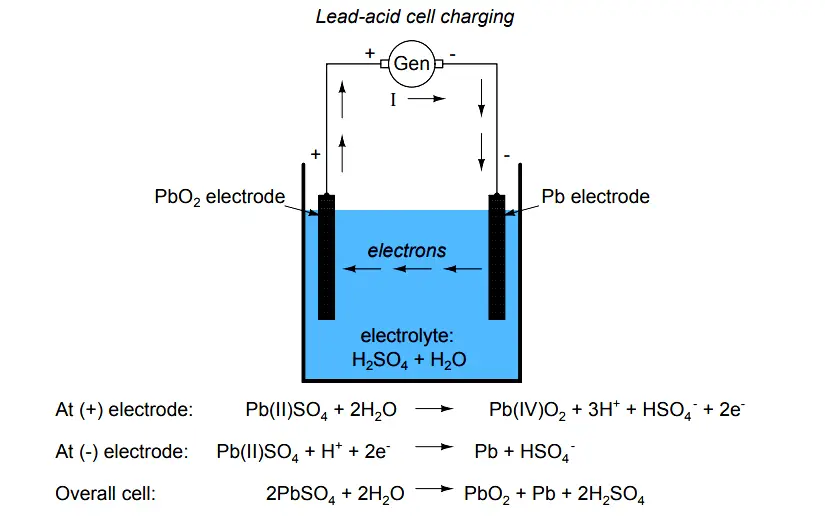
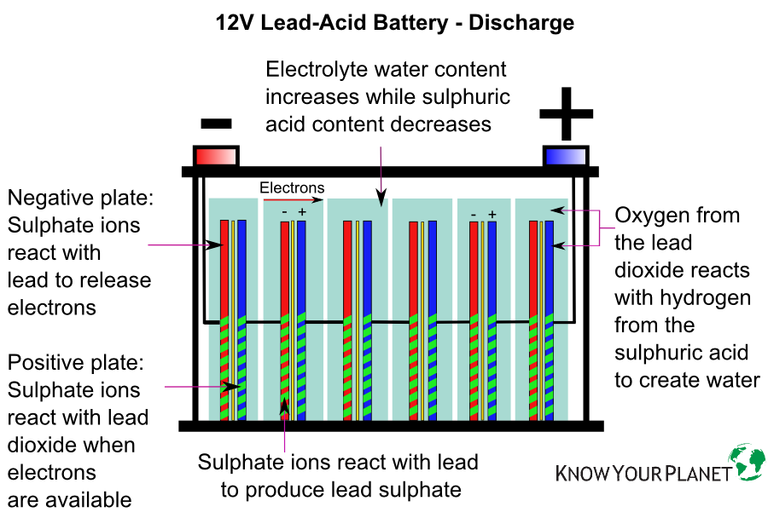
The cell diagram for the lead-acid. Here is a lead acid battery charger circuit using IC LM 317.The IC here provides the correct charging voltage for the battery.A battery must be charged with 1/10 its Ah value.This charging circuit is designed based on this fact.The charging current for the battery is controlled by Q1 ,R1,R4 and R5. Potentiometer R5 can be used to set the ... These larger crystals are unlike the typical porous structure of the lead electrode, and are difficult to convert back into lead. Voltage of lead acid battery upon charging. The charging reaction converts the lead sulfate at the negative electrode to lead. At the positive terminal the reaction converts the lead to lead oxide. A Guide To Lead-Acid Batteries Structure and Operation Most lead-acid batteries are constructed with the positive electrode (the anode) made from a lead-antimony alloy with lead (IV) oxide pressed into it, although batteries designed for maximum life use a lead-calcium alloy. The cell diagram for the lead-acid cell that is used in automobile and truck batteries is Pb (s) l PbSO4 (s) l H2SO4 (aq) l PbO2 (s), PbSO4 (s) l Pb (s) where the comma between PbO2 (s) and PbSO4 (s) denotes a heterogeneous mixture of the two solids and the right-hand lead electrode is nonreactive.
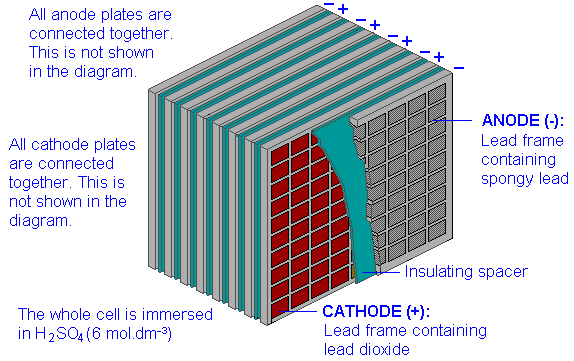
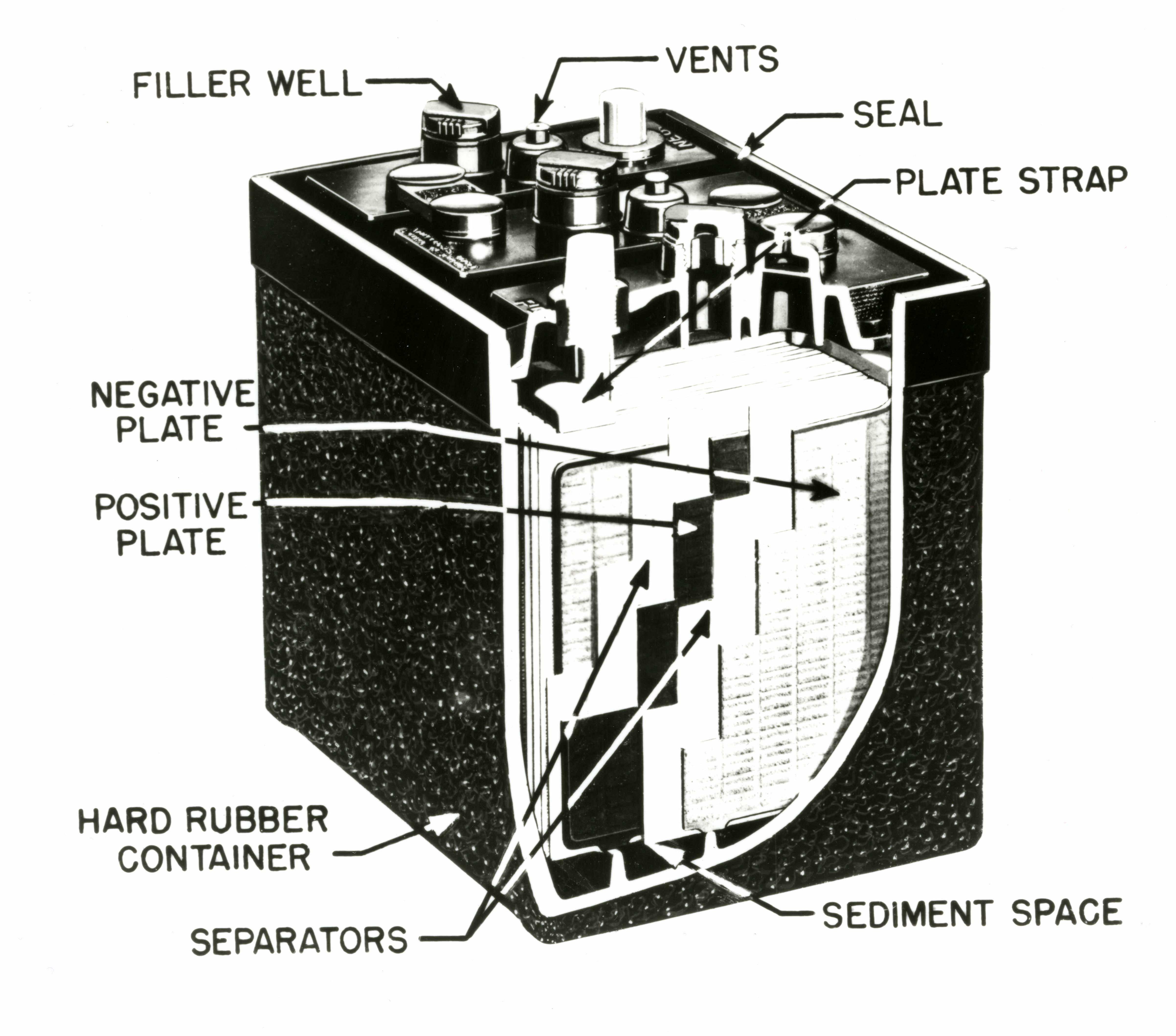
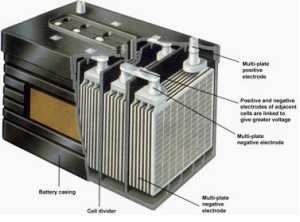
Plate - The plate of the lead-acid cell is of diverse design and they all consist some form of a grid which is made up of lead and the active material. The grid is essential for conducting the electric current and for distributing the current equally on the active material. The cell diagram for the lead-acid cell that is used in automobile and truck batteries is Pb (s) l PbSO 4 (s) l H 2 SO 4 (aq) l PbO 2 (s), PbSO 4 (s) l Pb (s) where the comma between PbO 2 (s) and PbSO 4 (s) denotes a heterogeneous mixture of the two solids and the right-hand lead electrode is nonreactive. The working principle of the lead acid cell can be explained with the help of a simple experiment. As you can see in the diagram above, two lead strips are immersed in the dilute sulfuric acid having specific gravity approximately equal to 1.200. One lead strip is the positive plate and the other lead strip is the negative plate. A lead-acid cell is a basic component of a lead-acid storage battery (e.g., a car battery). A 12.0 Volt car battery consists of six sets of cells, each producing 2.0 Volts. A lead-acid cell is an electrochemical cell, typically, comprising of a lead grid as an anode
Lead-Acid Battery Construction. The lead-acid battery is the most commonly used type of storage battery and is well-known for its application in automobiles. The battery is made up of several cells, each of which consists of lead plates immersed in an electrolyte of dilute sulfuric acid. The voltage per cell is typically 2 V to 2.2 V. The cell diagram for the lead acid cell that is used in automobile and truck batteries is pbs l pbso4 s l h2so4 aq l pbo2s pbso4s l pb s where the comma between pbo2 s and pbso4 s denotes a heterogeneous mixture of the two solids and the right hand lead electrode is nonreactive. A lead-acid cell basically contains two plates immersed in electrolyte (dilute sulphuric acid i.e. H 2 SO 4 of specific gravity about 1.28). The positive plate (anode) is made up of lead-peroxide (PbO 2) and the negative plate (cathode) is made up of sponge lead (Pb). Maintenance of lead acid cells : Care and maintenance of lead acid cells is most impotent. Other wise the batteries will get spoiled. The following points should be observed : 1)If the voltage of the lead acid cell comes down up to 1.8 volts per cell, the battery should not be used. It should be kept aside.
The things that I have in mind, In both sides the electrode material is. Separate every element that is in the same phase with a comma. Separate every element with different phases with a single bar. Separate the two half reactions with a single bar (as I find no salt bridge here) I am having a tough time learning cell notation.
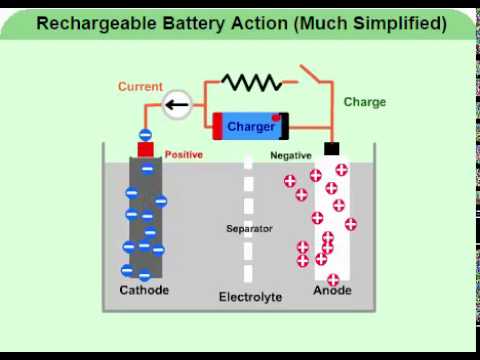
As the lead-acid cell discharges: PbSO 4 precipitates out and deposits on both the anode and the cathode.; H + from the electrolyte (H 2 SO 4(aq)) is being used to produce water at the cathode.; Concentration of H + will be decreased over time (concentration of H 2 SO (aq) decreases).; pH of the electrolyte (H 2 SO 4(aq)) will increase.; Connecting lead-acid galvanic cells in a series to make ...
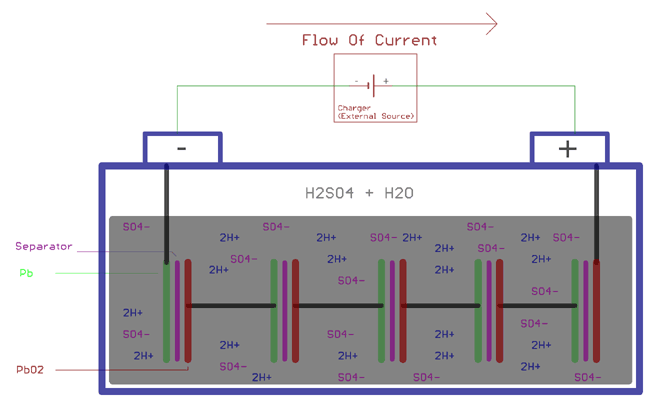
In this article we will discuss about the working of lead-acid battery with the help of diagram. When the sulphuric acid is dissolved, its molecules break up into hydrogen positive ions (2H +) and sulphate negative ions (SO 4 - -) and move freely.Now if two lead electrodes are immersed in this solution and connected to dc supply mains, the hydrogen ions being positively charged move ...
The cell diagram for the lead-acid cell that is used in automobile and truck batteries is. The comma between PbO2 (s) and )PbSO4 (s) denotes a heterogeneous mixture of the two solids. The right-hand lead electrode is nonreactive. Write the balanced equation for the net cell reaction. Look up standard potentials for the oxidation and reduction ...
MM9Z1_638 - 4-Cell Lithium Battery. Designs. 12 V Lead-Acid Battery Management System with LIN. Designs. 12 V Lead-Acid Battery Sensor with CAN. Designs. RD9Z1-638BJBEVM:High Voltage Battery Junction Box Reference Design. Designs. KIT9Z1J638EVM:Evaluation Kit - MM9Z1J638, Battery Sensor with CAN/LIN. Evaluation and Development Boards.
A Secondary Battery: The Lead Storage Battery. The electrodes of the cells in a lead storage battery consist of lead grids.The openings of the anodic grid is filled with spongy (porous) lead. The openings of the cathodic grid is filled with lead dioxide {PbO 2}.Dilute sulfuric acid {H 2 SO 4} serves as the electrolyte.When the battery is delivering a current, i.e.discharging, the lead at the ...
The cell diagram for the lead-acid cell that is used in automobile and truck batteries is Pb (s)|PbSO4 (s)|H2SO4 (aq)|PbO2 (s),PbSO4 (s)|Pb (s) The comma between PbO2 (s) and PbSO4 (s) denotes a heterogeneous mixture of the two solids. The right-hand lead electrode is nonreactive.
Every lead-acid battery is provided with datasheet for standard charge current and discharges current. Typically a 12V lead-acid battery which is applicable for the automotive application could be ranged from 100Ah to 350Ah. This rating is defined as the discharge rating with an 8 hour timing period.
The Daniell cell is a type of electrochemical cell invented in 1836 by John Frederic Daniell, a British chemist and meteorologist, and consists of a copper pot filled with a copper (II) sulfate solution, in which is immersed an unglazed earthenware container filled with sulfuric acid and a zinc electrode.
UltraBattery is a hybrid energy storage device, composed of a lead-acid cell and asymmetric supercapacitor that are connected in parallel with an internal noncontrolled circuit. Fig. 2.10 is the schematic diagram of the UltraBattery structure. In the UltraBattery, the positive plate of the lead-acid cell is made of lead dioxide, and the ...
Lead Acid Battery Diagram Container. This container part is constructed with ebonite, lead-coated wood, glass, hard rubber made of the bituminous element, ceramic materials, or forged plastic which are placed on the top to eliminate any kind of electrolyte discharge.
The sealed lead-acid battery consists of six cells mounted side by side in a single case. The cells are coupled together, and each 2.0V cell adds up to the overall 12.0V capacity of the battery.
The cell diagram for the lead-acid cell that is used in automobile and truck batteries is Pb(s) l PbSO4 (s) l H2SO4 (aq) l PbO2(s), PbSO4(s) l Pb (s) where the comma between PbO2 (s) and PbSO4 (s) denotes a heterogeneous mixture of the two solids and the right-hand lead electrode is nonreactive. a)Write a balanced equation for the net cell reaction: b)Look up standard potentials for the ...
• Identify the active materials in the lead-acid cell. • Describe the effects of temperature and discharge rate on battery capacity and life. • Identify industry and government standards for maintenance, testing, replacement, sizing, and installation of lead-acid batteries. • Identify the three most common applications of lead-acid ...
Lead-Acid battery. Lead-acid battery is from the secondary galvanic cells, It is known as a Car battery (liquid battery) because this kind of batteries is developed and becomes the most suitable kind of batteries used in cars, It consists of six cells are connected in series, Each cell produces E cell = 2 volt and the total cell potential of the battery is emf = 2 × 6 = 12 volts, This battery ...
The cell diagram for the lead-acid cell that is used in automobile and truck batteries is Pb (s) l PbSO4 (s) l H2SO4 (aq) l PbO2 (s), PbSO4 (s) l Pb (s) where the comma between PbO2 (s) and PbSO4 (s) denotes a heterogeneous mixture of the two solids and the right-hand lead electrode is nonreactive.
State two applications of lead acid storage cell. Last Answer : Applications of lead acid storage cell: 1. Lead acid storage cellis commonly used in automobiles. 2. To Supply current for electric vehicles 3. In Gas engine ignition 4. In telephone exchanges ... hospitals 7. In broad casting stations, power stations 8. For distribution work 9.
Feb 24, 2012 · A fully charged lead acid battery cell has voltage and specific gravity, 2.2 V and 1.250 respectively, and this cell is normally allowed to be discharged till the corresponding values become 1.8 V and 1.1 respectively. Maintenance of Lead Acid Battery
Plante plates or formed lead acid battery plates. Faure plates or pasted lead acid battery plates. Plante Plate Plante Process. In this process two sheets of lead are taken and immersed in dilute H 2 SO 4. When an current is passed into this lead acid cell from an external supply, then due to electrolysis, hydrogen and oxygen
The right-hand lead electrode is nonreactive. Write the balanced equation for the net cell reaction. equation: Look up standard potentials for the oxidation and reduction half-reactions, and then calculate the value of 𝐸∘cellEcell∘. 𝐸∘cell=Ecell∘=. VV. Calculate the value of Δ𝐺∘rxnΔGrxn∘. Δ𝐺∘rxn=ΔGrxn∘=.













0 Response to "38 the cell diagram for the lead-acid"
Post a Comment