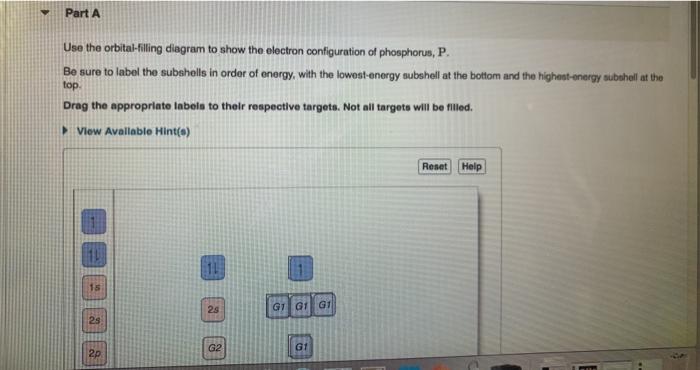
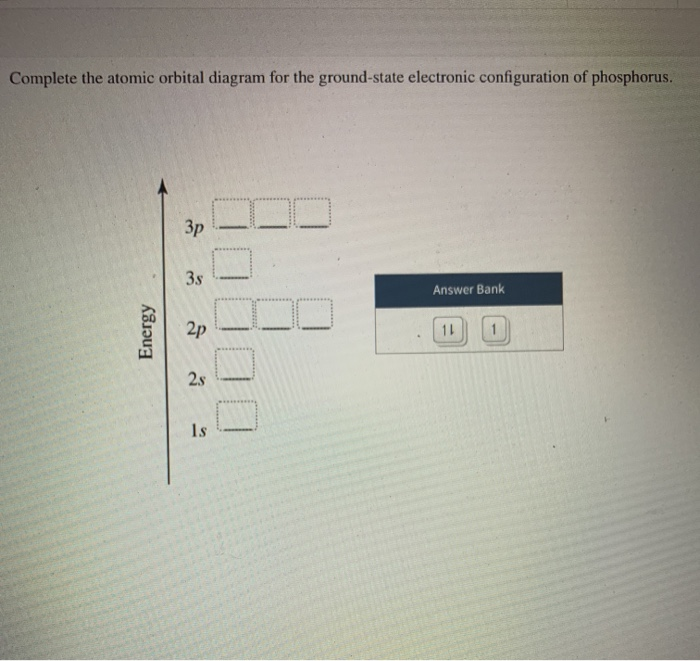
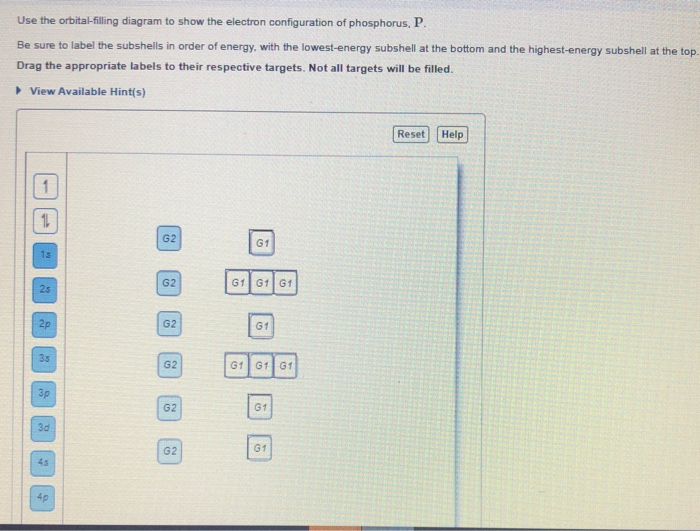
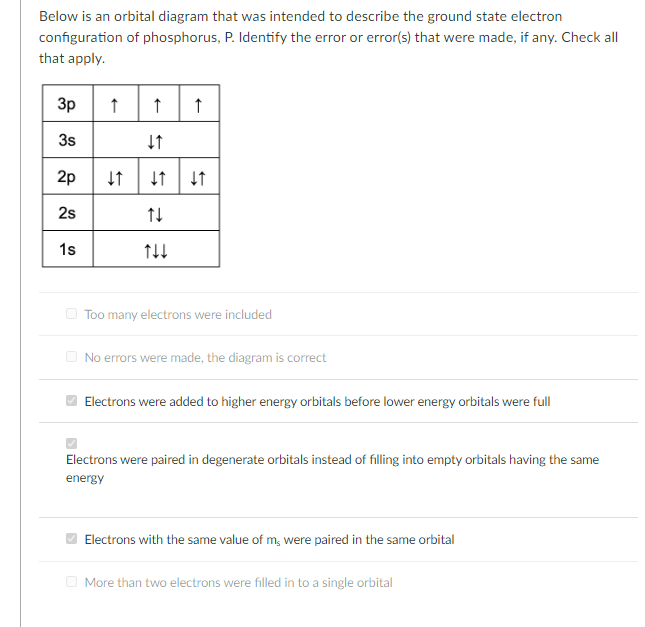
39 use the orbital-filling diagram to show the electron configuration of phosphorus, p.
What is the electron configuration and orbital diagram for a phosphorus atom? What are the four quantum numbers for the last electron added? Show Solution. The atomic number of phosphorus is 15. Thus, a phosphorus atom contains 15 electrons. The order of filling of the energy levels is 1s, 2s, 2p, 3s, 3p, 4s, . . . where 1s, 2s, and 2p are the occupied subshells, and the superscript "2" is the number of electrons in each of these subshells. Use the rules for determining electron configurations to write the electron configuration for P. Express your answer in complete form in order of orbital filling. For example, 1s 2 2s 2 should be entered as 1s 2 2s 2.
Orbital Diagram, electron configuration, and the noble gas notation for a zinc (Zn) atom.
Use the orbital-filling diagram to show the electron configuration of phosphorus, p.
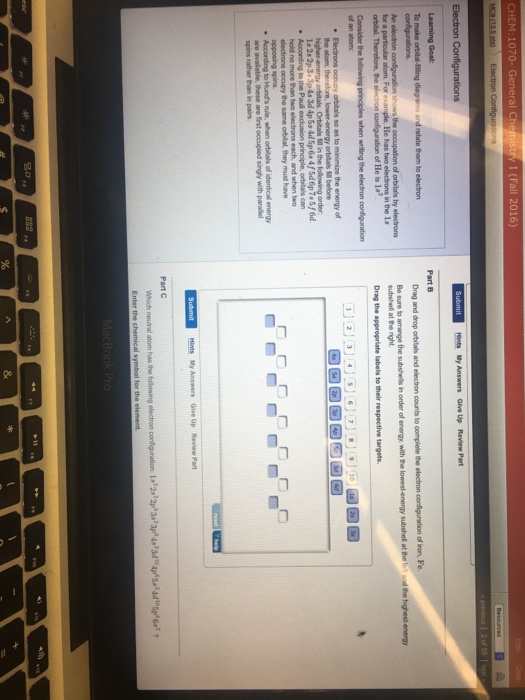
In writing the electron configuration for Phosphorus the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Phosphorous go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the ... Answer to: Use the orbital-filling diagram to show the electron configuration of phosphorus P By signing up, you'll get thousands of step-by-step...1 answer · Top answer: Atomic number of phosphorous is 15. Electronic configuration of phosphorous is as follows: P = 1s22s22p63s23p31s22s22p63s23p3 Orbital... Determine the number of electrons in a neutral phosphorus atom Reset Help Chemistry: M. X ViewPassignment Problemid=127565898 ③ 10126 Constants Periodic Table Drag and drop orbitals and electron counts to complete the electron configuration of iron, Fe.
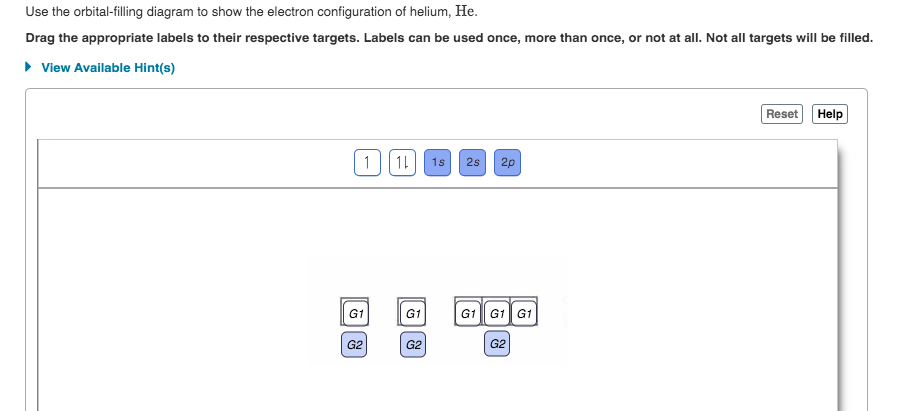
Use the orbital-filling diagram to show the electron configuration of phosphorus, p.. Depict the electron configuration for phosphorus using an orbital box diagram and noble gas notation. Give one possible set of four quantum numbers for each of the electrons beyond those of the preceding noble gas. Use the orbital-filling diagram to show the electron configuration of helium, He. ... Enter an abbreviated electron configuration for phosphorus: Express your answer in complete form, in order of increasing energy. [Ne] 3s2 3p3. Enter an abbreviated electron configuration for arsenic: Open 23+ pages part a orbital diagrams and longhand electron configuration answer in PDF format. 1 Orbital Diagrams And Electron Configuration Answers it also shows you how to find the 4 quantum numbers for an electron and how to write the electron configuration in addition to how to write the orbital notation or fill in the arrows in the electron configuration part ii add to favorites 40. Use the orbital-filling diagram to show the electron configuration of phosphorus, P. Be sure to label the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the Drag the appropriate labels to their respective targets. Not all targets will be filled.
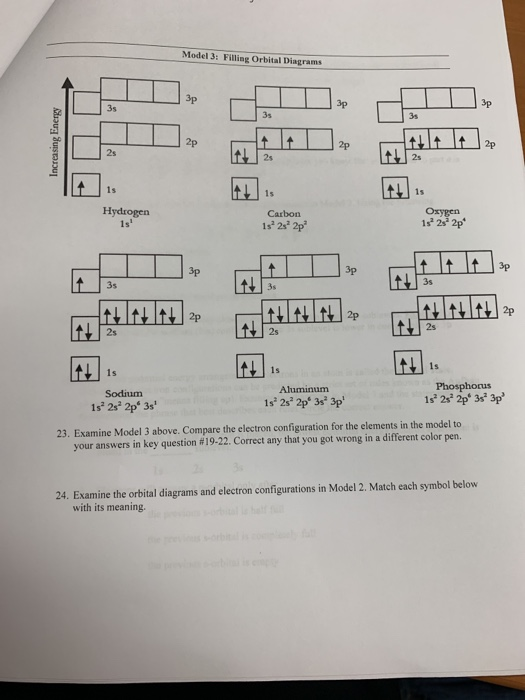
Box spin diagram of outer electron orbitals for the electron configuration of the 15 Phosphorus P 1s22s22p63s23p3 Ne3s 3p P pblock Gp5 Build the orbital diagram for the ion most likely formed by phosphorus. The rules for orbital filling diagrams. Energy 0 1 1 x 5. Now this is only one way we can draw the electron dot diagram for Oxygen. Draw ... This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n... What is the correct orbital diagram for phosphorus? Its electron configuration with regard to the electrons present each shell is 2,8,5 and in terms of molecular orbitals, its configuration is 1s2, 2s2 2p6, 3s2 3p3. The boxes represent sulfur's orbitals. Sulfur's electron configuration is 1s 2 2s 2 2p 6 3s 2 3p 4. Nov 14, · Draw an orbital diagram for boron. Draw an orbital diagram for scandium Show the orbital-filling diagram for N (nitrogen).
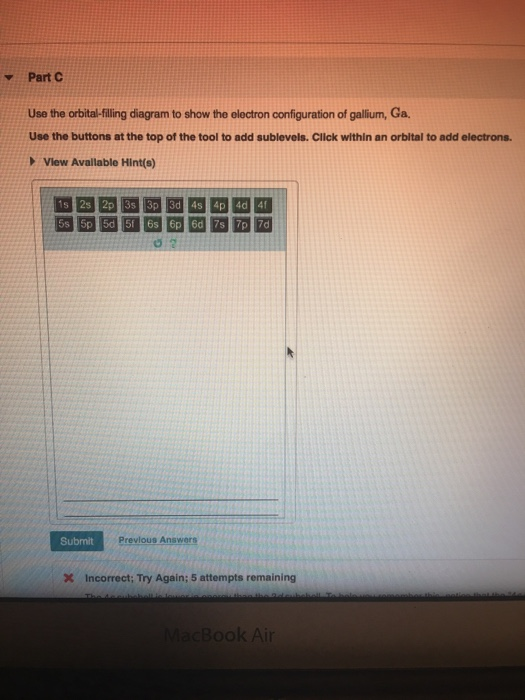
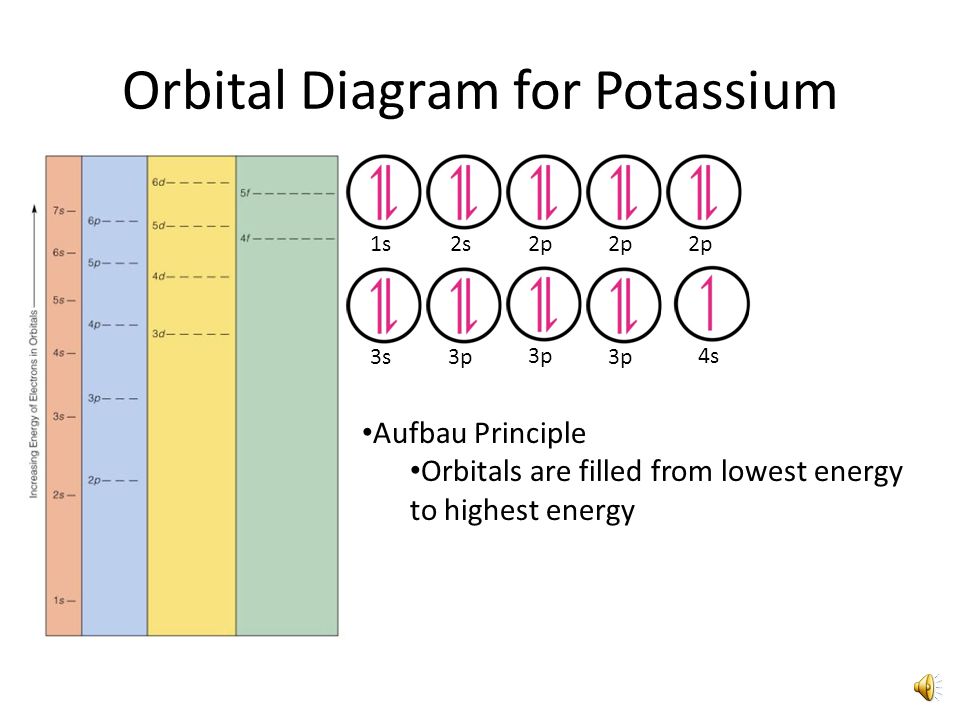
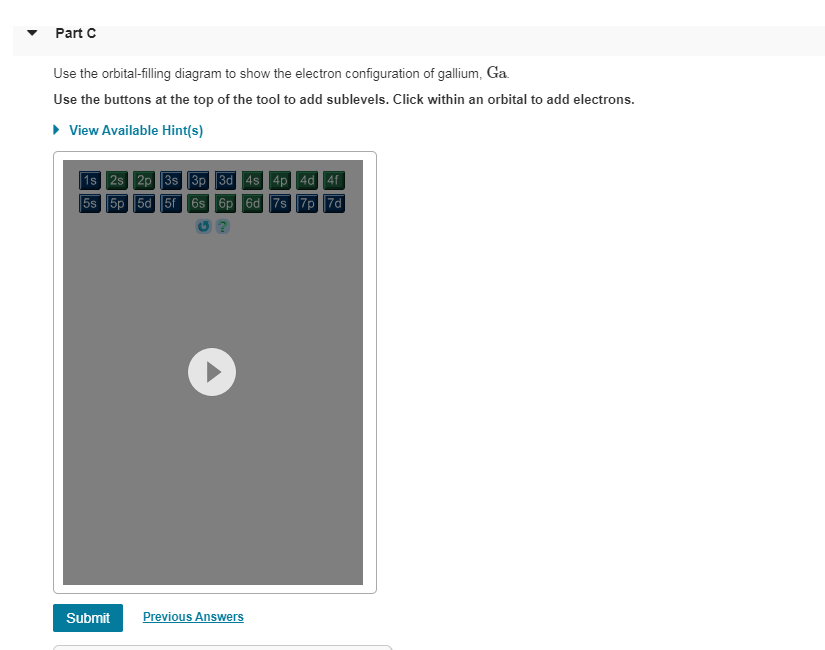
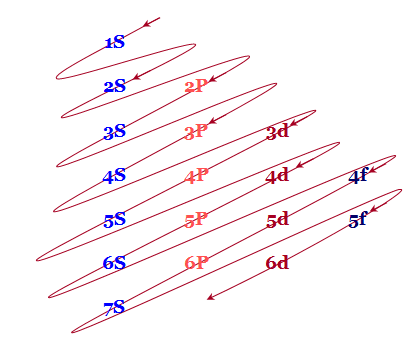
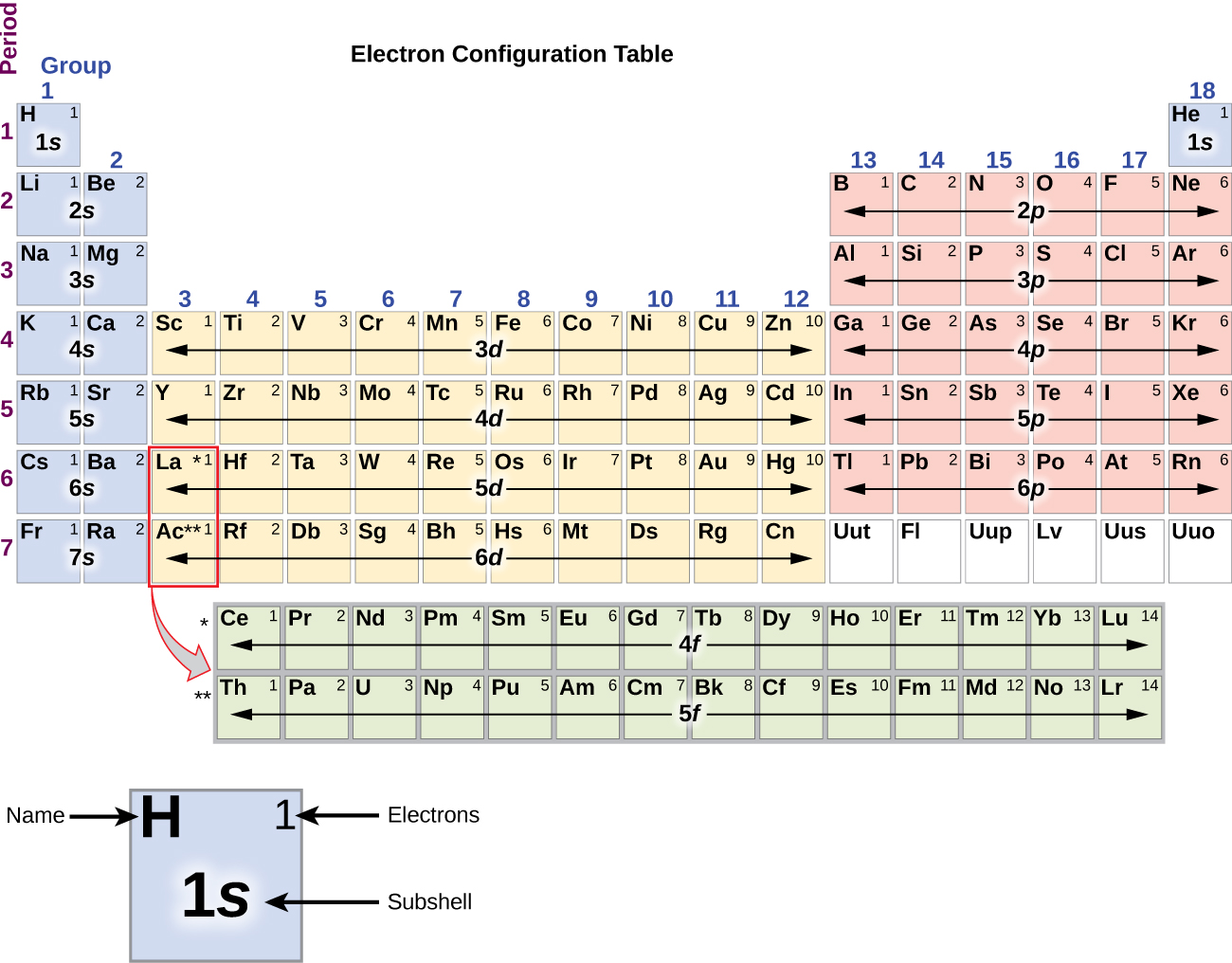
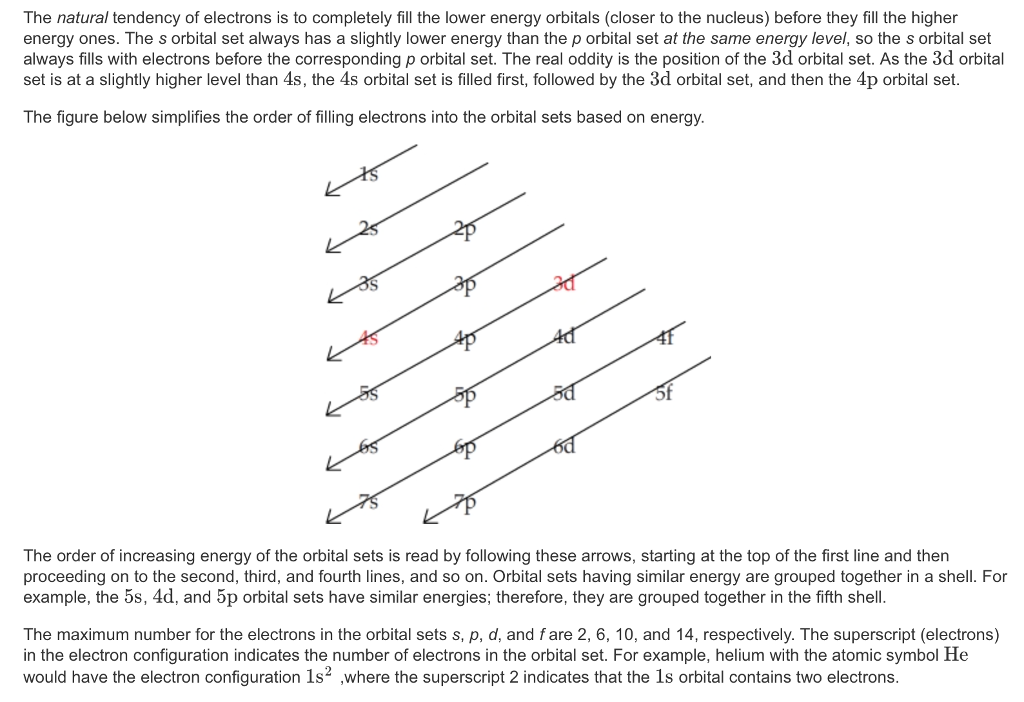
For example, after filling the 3p block up to Ar, we see the orbital will be 4s (K, Ca), followed by the 3d orbitals. This figure includes a chart used to order ... According to the Aufbau process, sublevels and orbitals are filled with electrons in order of increasing energy. Since the s sublevel consists of just one orbital, the second electron simply pairs up with the first electron as in helium. The next element is lithium and necessitates the use of the next available sublevel, the 2s.. The filling diagram for carbon is shown in the Figure below. The electron configuration for Gallium, Ga is 1s^2 2s^2 2p^6 3s^2 3p^6 3d^10 4s^2 4p^1 Gallium, Ga has 31 protons and 31 electrons. The superscripts represent the electrons present in each region of the periodic table. The sum of these superscripts should equal the atomic number for a neutral atom. The last electron is in the 4th period, in the p region and the first electron in that region. The key difference between orbital diagram and electron configuration is that the orbital diagram shows the electrons in arrows, indicating the spin of electrons. But, the electron configuration does not show details on the spin of electrons. The orbital diagram shows the arrangement of the electrons given by the electron configuration.
36 construct the orbital diagram of the f- ion. Construct the orbital diagram of each atom or ion. In writing the electron configurat ion for fluorine the first two electrons will go in the 1s orbital. The 24 electrons of a. Ion electron confugurat ion s. A neutral fluorine atom has 9 electrons.
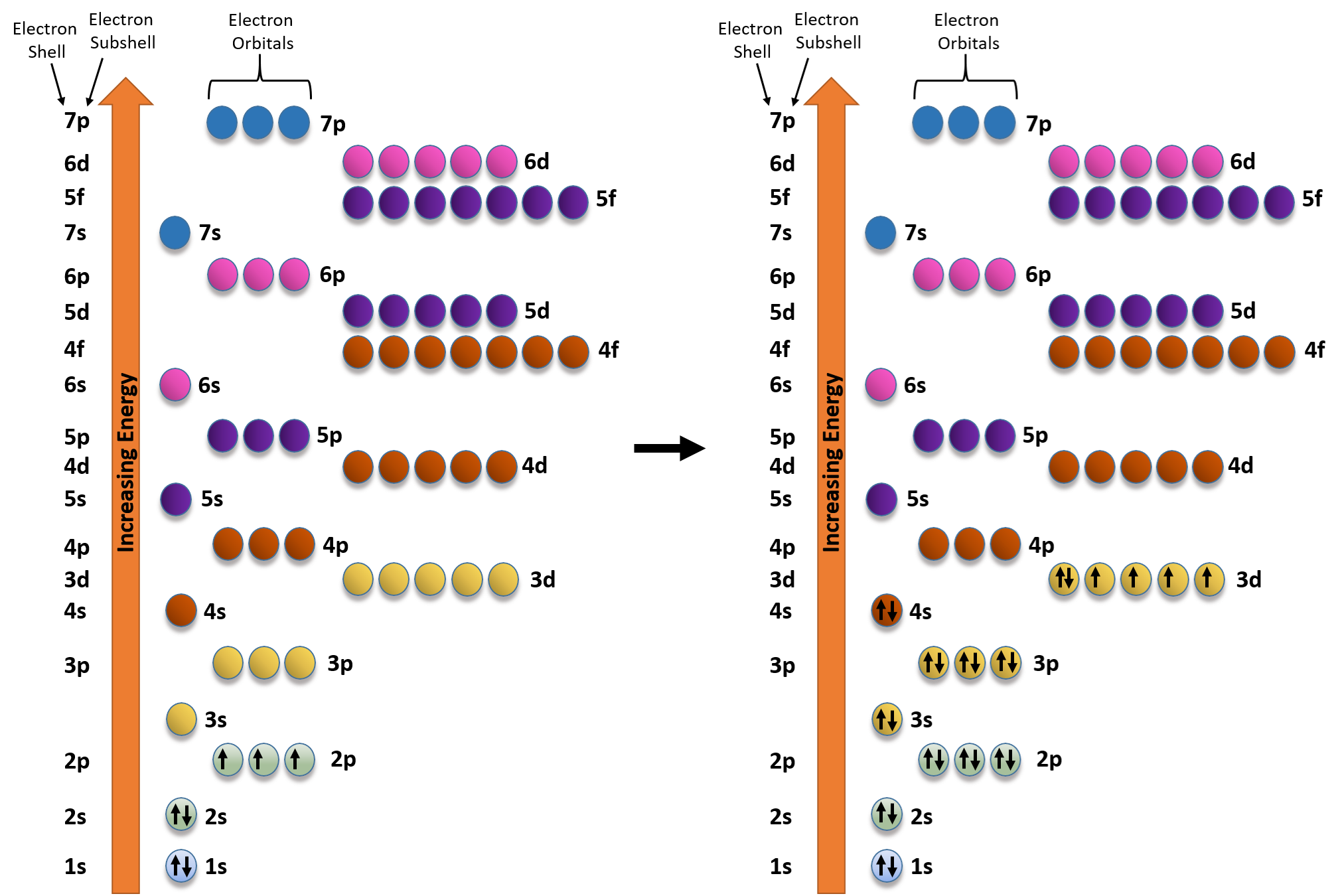
Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics.
What is the electron configuration and orbital diagram for a phosphorus atom? What are the four quantum numbers for the last electron added? Solution The atomic number of phosphorus is 15. Thus, a phosphorus atom contains 15 electrons. The order of filling of the energy levels is 1s, 2s, 2p, 3s, 3p, 4s, . . .
Jul 4, 2020 — The electron configuration chart for phosphorus, P, is given as follows;. 1s²2s²2p⁶3s²3p. ... Phosphorus, with the 3 partially filled p orbitals ...1 answer · 12 votes: Answer:1) The full electron configuration for phosphorus is 1s²2s²2p⁶3s²3p2) Phosphorus is paramagneticExplanation:1) To write the electron configuration, ...
Electron Arrangements Name There are three ways to indicate the arrangement of electrons around an atom: 1. Orbital Filling Diagram 02 Ex. 2, Electron Configuration 02 Ex. (gives the most information) Is (quicker to draw than orbital filling diagrams) Dot Pb 3. Electron Dot shows only the valence (outer energy level) electrons Oxygen atom Ex. 1.
Answer (1 of 3): You want a Walsh diagram for \mathrm{AH_3}. The molecular orbitals for \mathrm{PH_3} are on the right-hand side. The highest occupied orbital (HOMO) is \mathrm{2a_1}, but this diagram does not show it as occupied because it is using electrons for e.g. \mathrm{AlH_3}. (The reason ...
Electron Configurations. The content that follows is the substance of General Chemistry Lecture 26. In this lecture we continue the discussion of Quantum Numbers and their use in Electron Configurations as well as the relationship of electron configuration to the periodic properties of the elements.
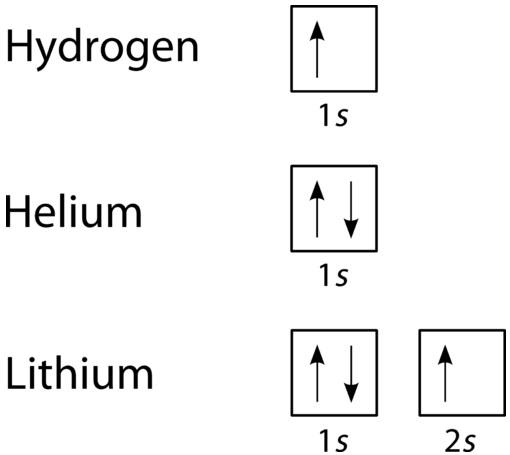
The orbital filling diagram of boron. I skipped past beryllium because I was getting bored. The electron configuration of boron is 1s²2s²2p¹, which means that there are two electrons in the 1s orbital, two electrons in the 2s orbital, and one electron in the 2p orbitals. This gives us an orbital filling diagram of:
The nex six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next two electrons in the 3s. Since the 3s if now full we'll move to the 3p where we'll place the remaining electron. Therefore the Aluminium electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 1.
May 30, 2020 — The electron configuration of an atom indicates the number of valence ... We can clearly see that p orbitals are half-filled as there are ...
Jan 21, 2021 — Phosphorous is a chemical element that has an atomic number of 15. Its electron configuration with regards to electrons present in each shell is ...
You are watching: Use the orbital-filling diagram to show the electron configuration of phosphorus, p. Electron Configuration. Electron configurations are the summary of where the electrons are around a nucleus. As we learned earlier, each neutral atom has a number of electrons equal to its number of protons.
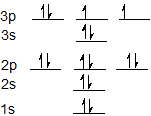
Question: Use the orbital filling diagram to show the electron configuration of phosphorus, P. Be sure to label the subshells in order of energy, with the ...
Orbitals can hold no more than two electrons each. For example, the s subshell contains one orbital and can hold a maximum of two electrons, while the p subshell contains three orbitals and can hold a maximum of six electrons. Use the orbital-filling diagram to show the electron configuration of aluminum, AlAl.
Electrons fill orbitals in a way to minimize the energy of the atom. Therefore, the electrons in an atom fill the principal energy levels in order of increasing energy (the electrons are getting farther from the nucleus). ... The electron configuration for phosphorus is 1s 2 2s 2 2p 6 3 s 2 3p 3 and the orbital diagram is drawn below. Orbital ...
Part A Use the orbital-filling diagram to show the electron configuration of phosphorus,. Be sure to label the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. Drag the appropriate labels to their respective targets. Not all targets will be filled.
Determine the number of electrons in a neutral phosphorus atom Reset Help Chemistry: M. X ViewPassignment Problemid=127565898 ③ 10126 Constants Periodic Table Drag and drop orbitals and electron counts to complete the electron configuration of iron, Fe.
Answer to: Use the orbital-filling diagram to show the electron configuration of phosphorus P By signing up, you'll get thousands of step-by-step...1 answer · Top answer: Atomic number of phosphorous is 15. Electronic configuration of phosphorous is as follows: P = 1s22s22p63s23p31s22s22p63s23p3 Orbital...
In writing the electron configuration for Phosphorus the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Phosphorous go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the ...












0 Response to "39 use the orbital-filling diagram to show the electron configuration of phosphorus, p."
Post a Comment