37 orbital diagram for germanium
9: Do the complete orbital diagram for germanium at the bottom of the page. (Abbreviated is fine.) a: Co b: Ca c: Cl d: W e: Hg f: Sn g: Ag h: Ar i: Am (element 95) 11: Do orbital diagrams for each of the following elements, then determine the number of unpaired electrons. Orbital Diagrams Chemistry Tutorial. Key Concepts. An orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom. A box, line, or circle, is drawn to represent each orbital in the electron configuration (using the Aufau Principle to order the orbitals and hence...
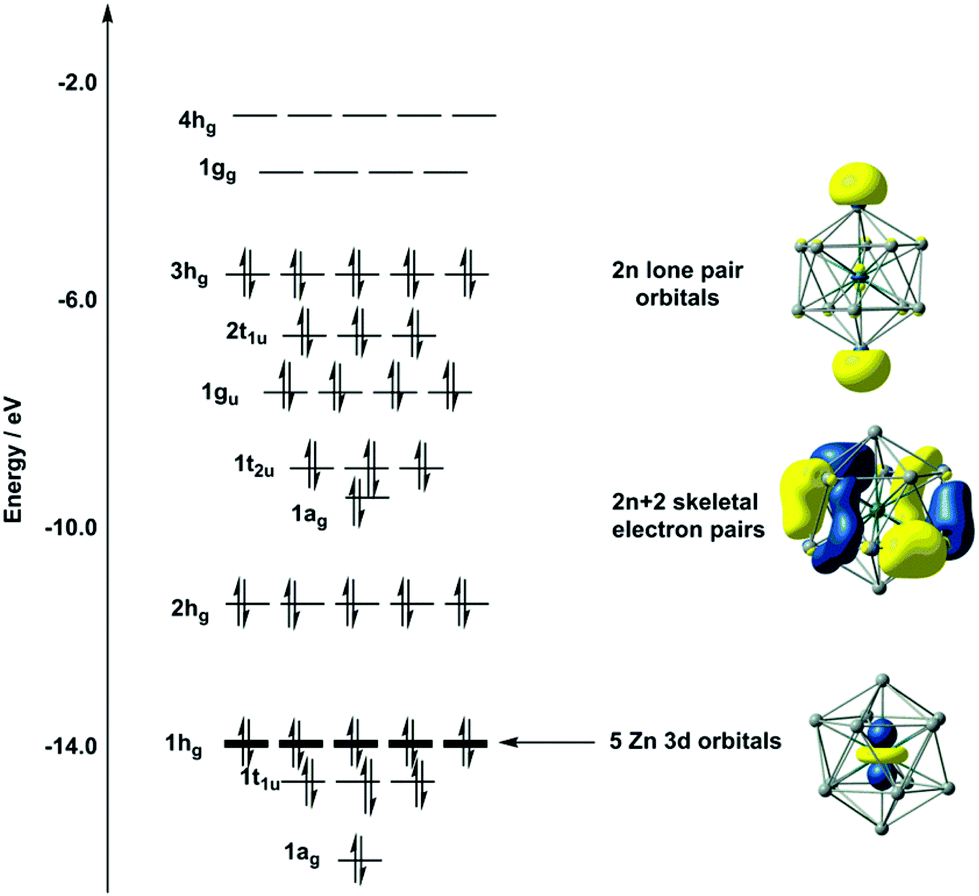
Metallic Germanium and Metallization Chemistry Germanium is a semiconductor. In fact, germanium was the first material to be fabricated into practical The molecular orbital (MO) treatment of their bonding also affords valuable insights. In this approach the geometrical distortions are explained on...

Orbital diagram for germanium
A bare molecular orbital diagram is presented and you must drag the correct orbitals and labels onto the diagram. The diagram is then completed by filling the energy levels with the correct number of electrons. The following molecules are currently available: Molecules of the First Row An orbital diagram, or orbital filling diagram, is a type of notation which illustrates an atom's electron distribution and electron spin within orbitals. The Basics of Orbital Diagrams. There are different types of orbitals, that all have different energy levels. These orbitals are filled with electrons (the... Check out here for Germanium Electron Configuration (Ge) with Orbital Diagram which is provided here for the users with complete details. Germanium Electron Configuration: Ge (Germanium) is a chemical element that has a chemical symbol Ge. The atomic number of Germanium is 32.
Orbital diagram for germanium. Hey Guys, ​ I was wondering if there are some databases for molexular orbital diagrams of more unusual compounds like phosphaalkenes or sulfur nitrides. I wanted to include some in a presentation ​ thanks for any help! Germanium is a chemical element with the symbol Ge and atomic number 32. It is a lustrous, hard-brittle, grayish-white metalloid in the carbon group... here we have to write Orbital Diagram for ground state Germany. The atomic number for Germanium is 32 here will follow this chart to how to Parents. The electrons in different shells. 1st 1 will be one is two electrons of opposite spain, one is two, then two is two. Molecular orbital (MO) theory describes the behavior of electrons in a molecule in terms of combinations of the atomic wavefunctions. The resulting molecular orbitals may extend over all the atoms in …
Orbital. This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram... For group, there should be a fully filled s sublevel and two electron in the outermost p sublevel. Based on how covalent bonds form with singly filled orbitals, in group14, there are only 2 singly filled orbitals respectively, how can they form the expected 4 bonds? Or does the electron from s move to p to give 4 singly filled orbitals? If that is the case, why does this happen for no reason? 3 Mendeleev's Predicted Properties of Germanium ("eka Silicon") and Its Actual Properties Table 8.1 Predicted Properties of eka Silicon(E) Actual Properties of Germanium (Ge) Property 15 A vertical orbital diagram for the Li ground state no color = empty light = half-filled Sublevel energy increases...
Lewis structure Potassium Electron Diagram Argon, symbol, chemical Element, angle png. Electron configuration Germanium Electron shell Bohr model Valence electron, copper shell, chemical Element, electron png. Orbital Energy Diagram and Atomic Electron Configuration Tool. As for an actual diagram (per Wiki. ) is above. You can also find the electron configuration on both websites. For an overview and better understanding of electron configuration and orbital diagrams please visit the PDF below( although... The following molecular orbital diagram may be used for problems 32-46. For oxygen and fluorine, the σ2p orbital should be 46. Assuming that the molecular orbital energy diagram for a homonuclear diatomic molecule applies to a Silicon and germanium are examples of this type of semiconductor. Is germanium paramagnetic or diamagnetic.
Electron Configuration Tutorial Germanium. Для просмотра онлайн кликните на видео ⤵. Orbital Diagrams and Electron Configuration - Basic Introduction - Chemistry Practice ProblemsПодробнее.
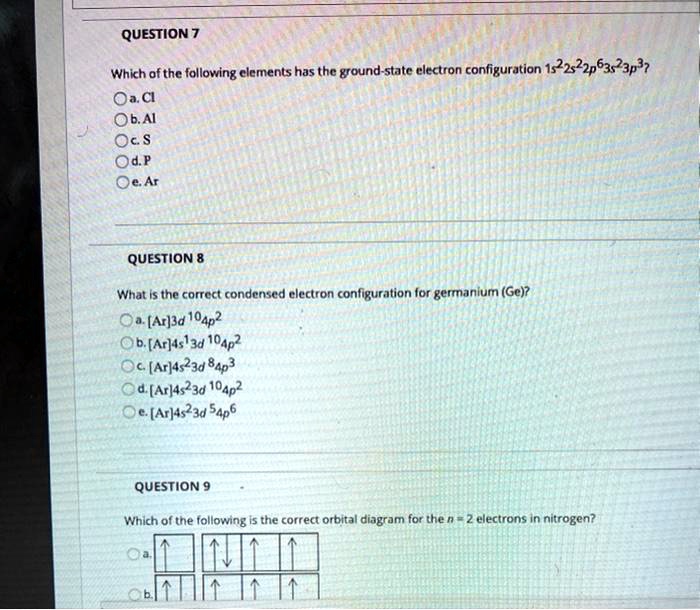
Choose The Orbital Diagram That Represents The Ground. Consider the bohr model of the hydrogen atom. A possible set of quantum numbers for the last electron added to complete an atom of germanium in its ground state is a. Since 1s can only hold two electrons the next 2 electrons for n...
Orbital diagrams make use of a box, circle, or line for each orbital in the energy level. An arrow is used to represent an electron and its spin. Figure 8.6 A vertical orbital diagram for the Li ground state. 8-14. Building Orbital Diagrams. The aufbau principle is applied - electrons are always placed...
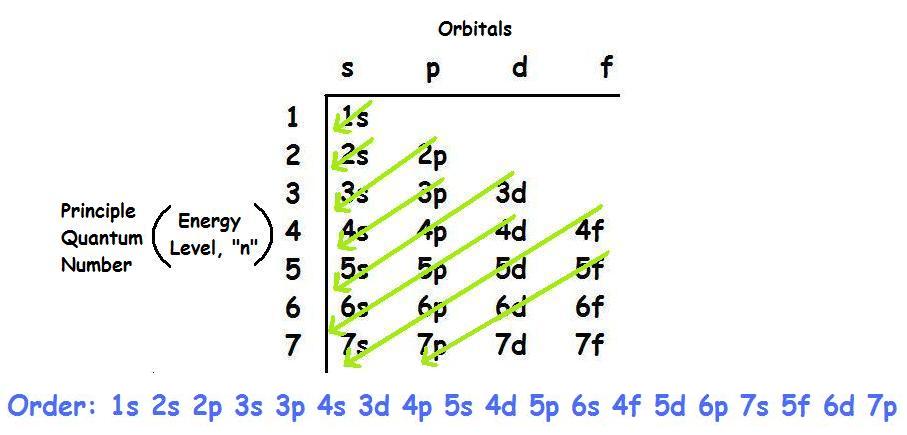
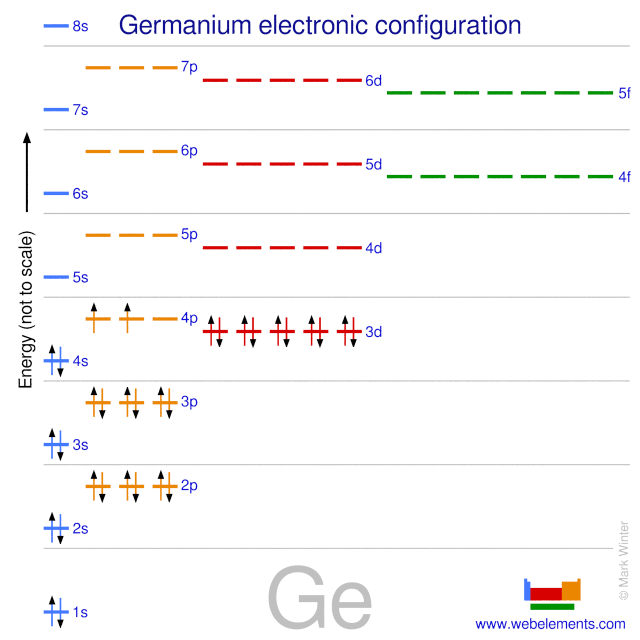
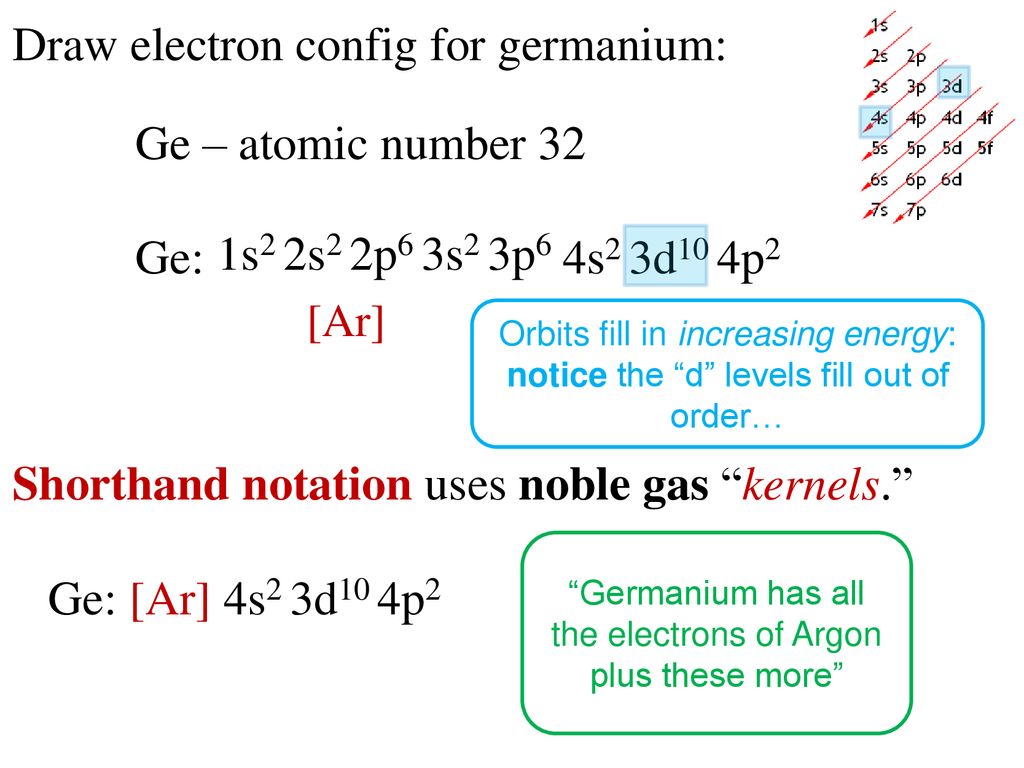
The electron configurations and orbital diagrams for the rest of the elements of the second period show the The last electrons to be added to an orbital diagram for the atoms of the transition metal elements go [Kr] 5s 2 b. With an atomic number of 32, germanium has 32 electrons. The noble gas.
An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with up and down arrows to represent the electrons in each orbital. Refer to the related link to see an illustration of an orbital diagram for aluminum.
Germanium is a semiconductor. The pure element was commonly doped with arsenic, gallium or other elements and used as a transistor in thousands of electronic applications. Today, however, other semiconductors have replaced it. Germanium oxide has a high index of refraction and dispersion.
Write an orbital diagram for silicon. SOLUTION Since silicon is atomic number 14, it has 14 electrons. Draw a box for each orbital, putting the lowest-energy orbital (1s) on the far left and proceeding to orbitals of higher energy to the right. The electron configuration of a germanium atom is.
To draw an orbital diagram for an uncharged atom, Step 1 Write the complete electron To draw the orbital diagram, we draw a line for each orbital of each sublevel mentioned in the element at the end of the previous row is argon, so putting Ar in brackets describes germanium's first 18 electrons.
Only RUB 220.84/month. Orbital Diagrams, Electron Configurations, Electron Configurations, Bohr Models. Germanium (electron configuration).
I need to construct the molecular orbital diagram for the hypothetical species Li4, which has the following geometrical arrangement: https://preview.redd.it/npsjre5pch571.png?width=197&format=png&auto=webp&s=c2a7948c2efa04a975bee1db722838fae7482456 The first step is to identify the point symmetry group. In this particular case, we consider that there is only one axis of rotation of order four (actually, other symmetry elements can be observed, but this is a previous consi...
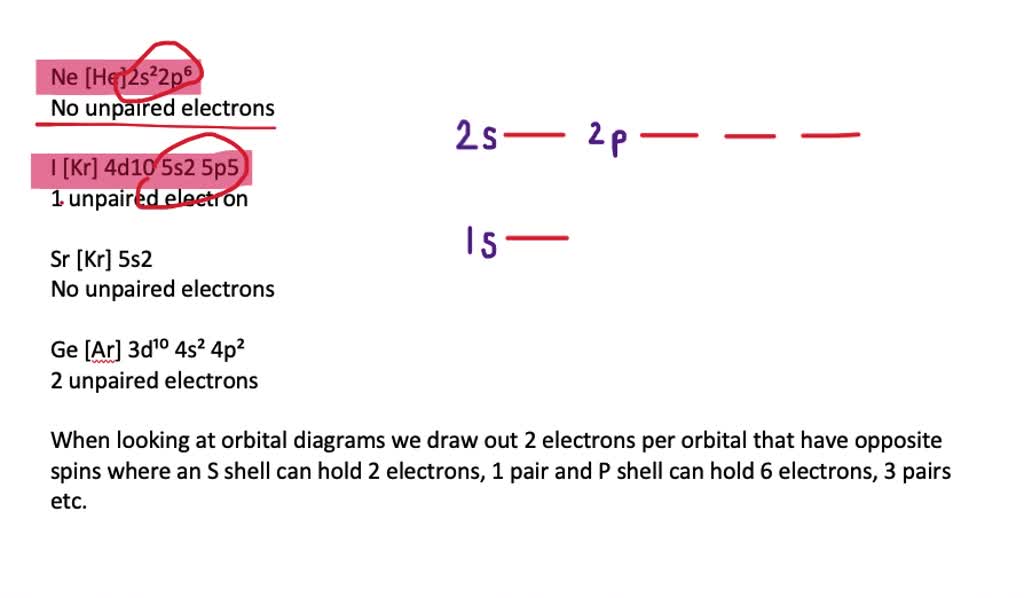
write orbital diagrams for the valence electrons and indicate the number of unpaired electrons for 2
Germanium is a gray-white semi-metal, and in its pure state is crystalline and brittle, retaining its lustre in air at room temperature. It is a very important semiconductor material. Zone-refining techniques have led to production of crystalline germanium for semiconductor use with an impurity of only one part in...
Electronic configuration of the Germanium atom. Valence electrons. Orbital diagram. Ge (Germanium) is an element with position number 32 in the periodic table. Located in the IV period. Melting point: 937.4 ℃. Density: 5.32 g/cm3.
Germanium Orbital Diagram - schematron.org. Economy. Details: A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular...
I’ve been tasked with drawing rhe MO diagram for Sulfure Oxide and I’m not sure about the energies of the relatove orbitals. Since Oxygen is more electronegative I expect the 2s and 2p orbitals to have much lower energy than the 3s and 3p orbitals sulfur has. But the energy difference would be really high then. So I’m not sure what 2 orbitals combine to form the sigma 3s or sigma* 3s orbital. The difference in energy kevels confuses me as every example I’ve done has the same orbitals (2s,2p’s) c...
Orbital diagrams are a visual way to show where the electrons are located within an atom. Orbital diagrams must follow 3 rules How to draw the Bohr-Rutherford Diagram for Germanium. The order of filling makes Bohr-Rutherford Diagrams for Elements ...
I stack silver. But the primal urge for all things shiny have made this ape grab a couple others as well. Started looking at 5 year charts of strategic/base metals. Two of them jumped out at me and I cannot find a thing about WHY they behaved as they did. If you look at Aluminum, it is prone to these dramatic spikes; ie, it skyrockets, and then plummets, quickly. Alot. Why? World reserves of aluminum have never been in danger, it is heavily recycled, and I cannot find anything solid about ...
Sorry if it's a dumb question, I'm having trouble understanding
On Wulf's diagram the orientations form plateau in the vicinity of (110). The surfaces with such orientations An analysis of the orbital populations shows that indeed the nature of the hybridization of. The potential surface for unreconstructed {001} face of germanium has been calculated by the...
Check out here for Germanium Electron Configuration (Ge) with Orbital Diagram which is provided here for the users with complete details. Germanium Electron Configuration: Ge (Germanium) is a chemical element that has a chemical symbol Ge. The atomic number of Germanium is 32.
An orbital diagram, or orbital filling diagram, is a type of notation which illustrates an atom's electron distribution and electron spin within orbitals. The Basics of Orbital Diagrams. There are different types of orbitals, that all have different energy levels. These orbitals are filled with electrons (the...
A bare molecular orbital diagram is presented and you must drag the correct orbitals and labels onto the diagram. The diagram is then completed by filling the energy levels with the correct number of electrons. The following molecules are currently available: Molecules of the First Row
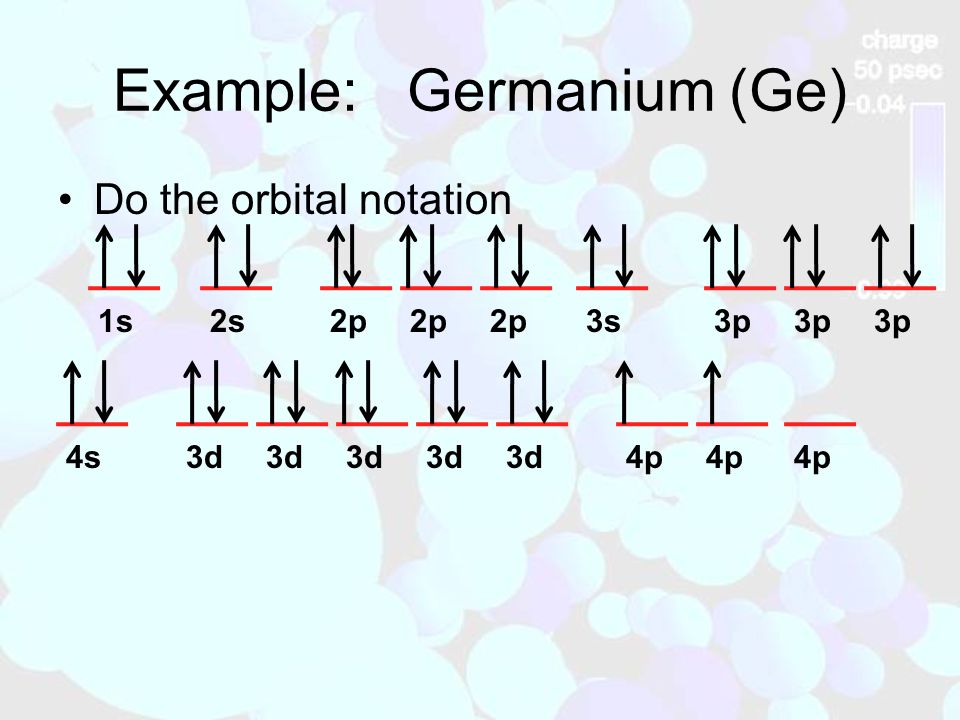

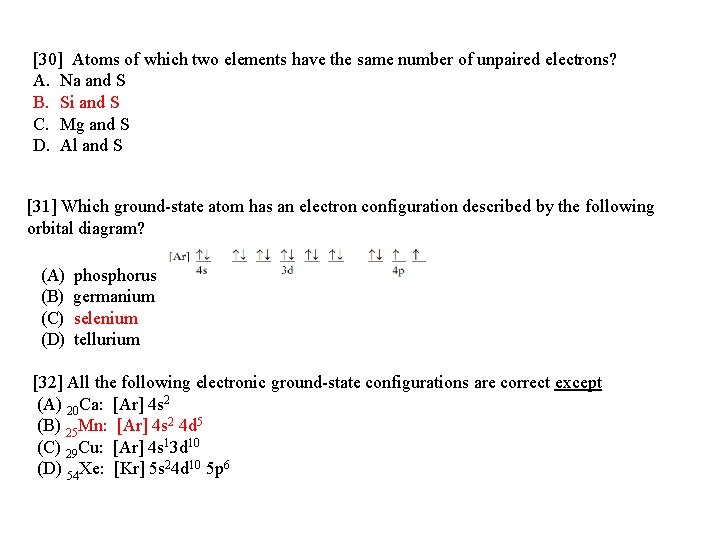
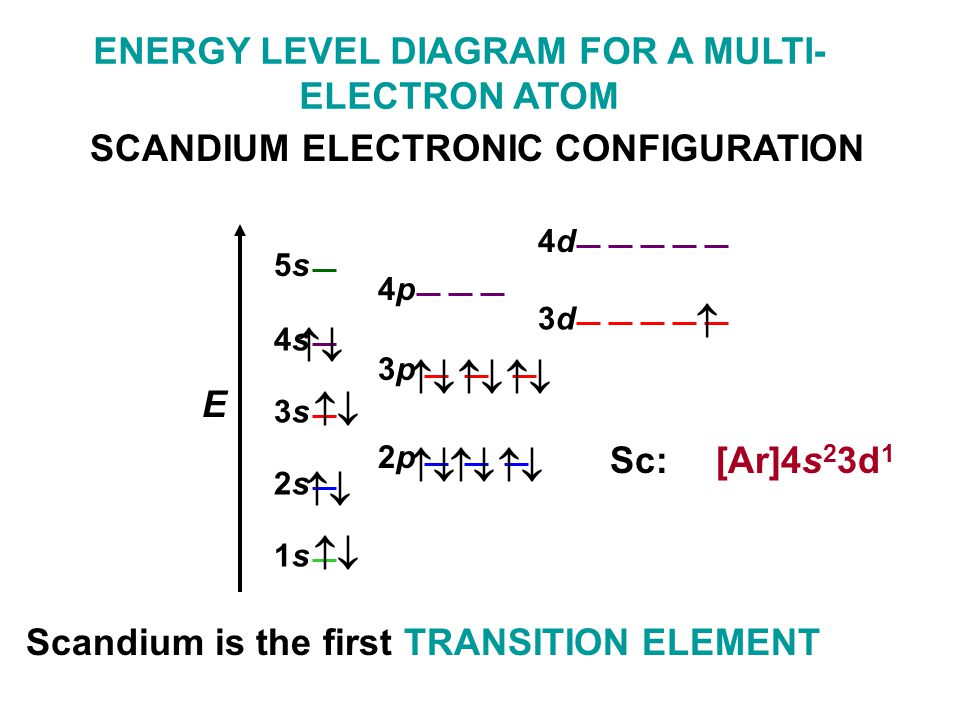



















0 Response to "37 orbital diagram for germanium"
Post a Comment